Compounds with aggregation-induced luminescence function, preparation methods and applications thereof
A technology of aggregation-induced luminescence and compounds, applied in the field of compounds with aggregation-induced luminescence function, can solve the problems of low labeling efficiency, small Stokes shift, poor photostability, etc., to improve detection sensitivity and facilitate post-processing operation , the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
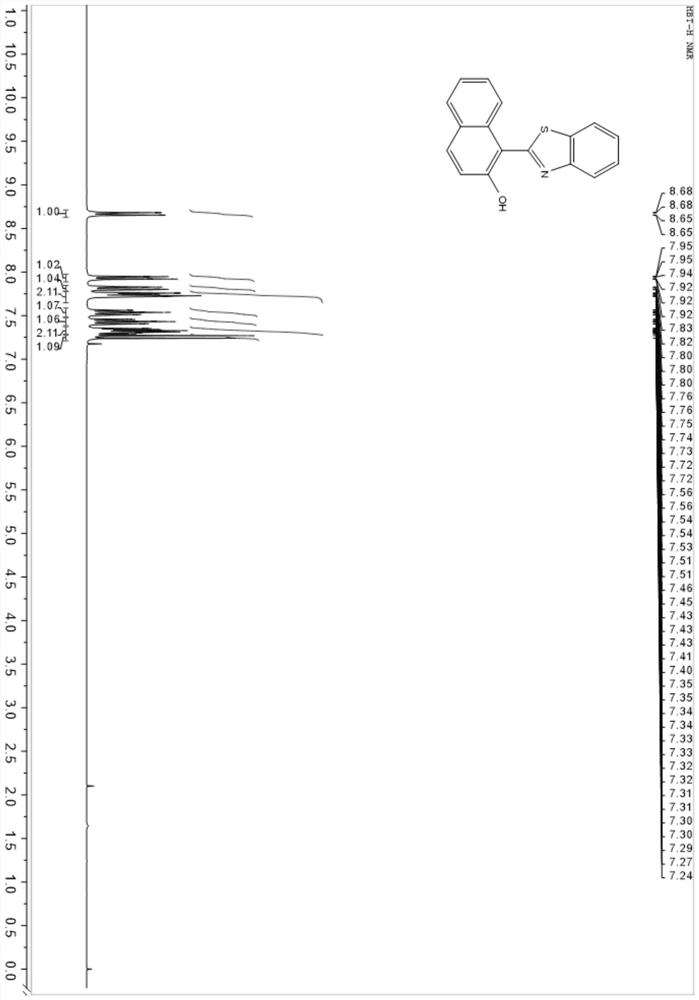
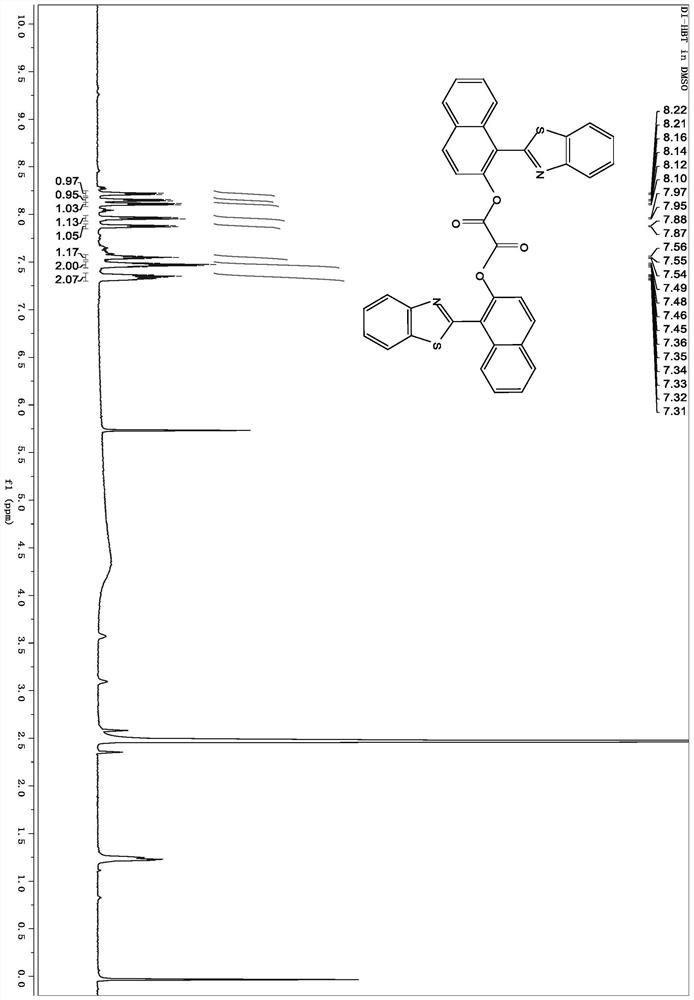
[0045] The invention provides a method for preparing an aggregation-induced luminescent compound. The 2-hydroxy-1-naphthalene carboxaldehyde and the compound of formula II are condensed to form an intermediate with a Schiff base structure of formula III; . Further and oxalyl chloride, bromomethylbenzene boronate pinacol ester, nitric acid, Br 2 Derivatization reactions give compounds of formula I. figure 1 It is the basic roadmap for the synthesis of the aggregation-induced luminescent compound prepared in the present invention. The structures of various compounds are as follows:
[0046]
[0047] II, where X=O or S.
[0048]
[0049] III, where X=O or S.
[0050]
[0051] IV, where X=O or S.
Embodiment 1
[0053] Mix 1 mmol of 2-hydroxy-1-naphthalenecarboxaldehyde and 1.05 mmol of 2-aminothiophenol in 10 mL of absolute ethanol, heat to reflux under nitrogen protection, and react for 8 to 12 hours, or monitor the progress of the reaction with a thin-layer chromatographic plate. The reaction was stopped after the reaction of the raw materials was completed. After the temperature of the reaction solution is lowered to room temperature, the intermediate of the Schiff base structure is obtained; after the intermediate is purified by recrystallization from ethanol, the pale yellow solid is collected by filtration to obtain a light yellow solid which is directly used in the next oxidation reaction, and the oxidant 2,3-dichloro-5, 6-Dicyano-p-benzoquinone (DDQ) was heated to reflux in toluene and reacted for 4 hours to obtain the crude product of compound 1 with aggregation-induced luminescence function. The obtained crude product was concentrated, passed through a silica gel chromatogr...
Embodiment 2
[0059] 1mmol 2-hydroxy-1-naphthalene carboxaldehyde and 1.05mmol 2-aminothiophenol were mixed in 10mL absolute ethanol, heated to reflux under nitrogen protection, and reacted for 8 hours. The reaction progress was monitored with a thin layer chromatography plate. After the reaction of the raw materials was completed Stop the reaction. After the temperature of the reaction solution is lowered to room temperature, the intermediate of the Schiff base structure is obtained; the intermediate is obtained by recrystallization and purification in ethanol, and the oxidant 2-iodoylbenzoic acid is heated and refluxed in toluene, and reacted for 2 hours to obtain an aggregation-induced luminescence. The crude product of functional compound 1 was concentrated, and the crude product obtained was passed through a silica gel chromatography column and eluted with ethyl acetate / n-hexane (volume ratio 1 / 15) to obtain 113 mg of compound 1 with a yield of 41%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com