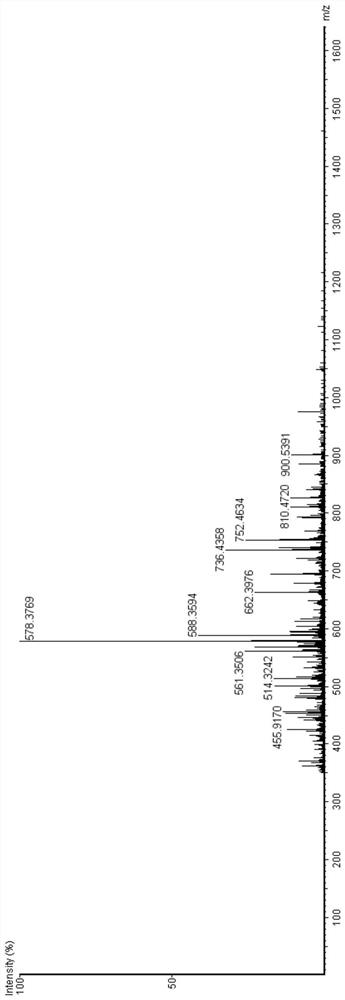
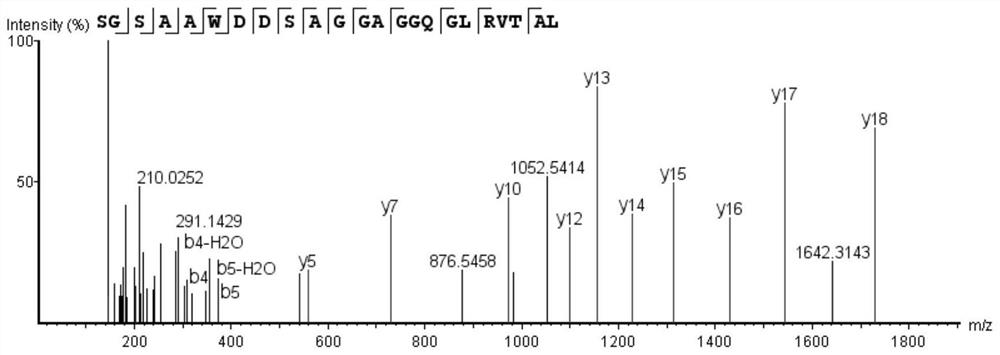
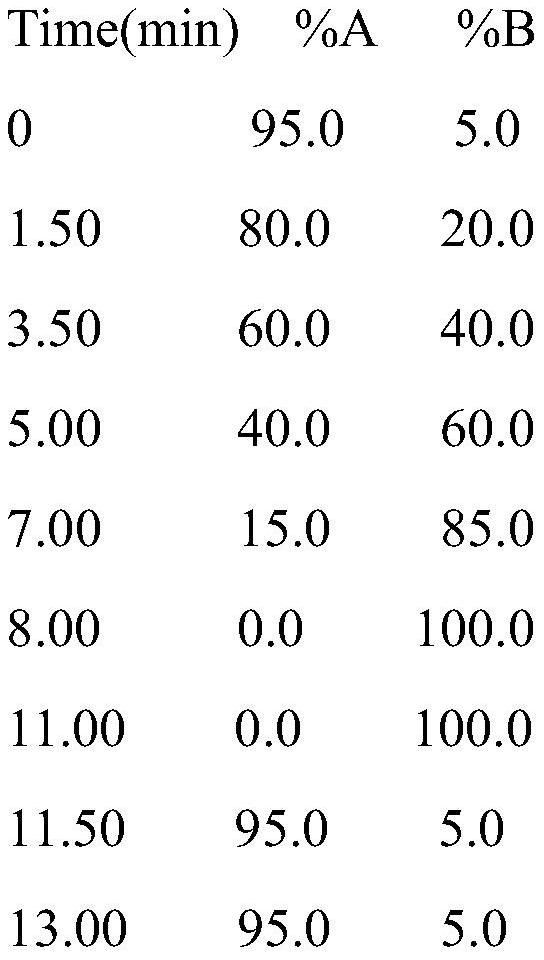
Bioactive peptide SGSAAWDDSAGGAGGQGLRVTAL as well as preparation method and application of bioactive peptide SGSAAWDDSAGGAGGQGLRVTAL
A bioactive peptide, active technology, applied in the field of protein to achieve the effect of improving quality, improving ability, and reducing the incidence of the body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The artificial synthesis of embodiment 1 bioactive peptide SGSAAWDDSAGGAGGQGLRVTAL
[0033] 1. Synthesis of Bioactive Peptides
[0034] 1. Weigh 3g of RINK resin (degree of substitution: 0.3mmol / g) into a 150ml reactor and soak with 50ml of dichloromethane (DCM).
[0035] After 2.2 hours, the resin was washed with nitrogen-dimethylformamide (DMF) 3 times the volume of the resin, and then drained. This was repeated four times, and the resin was drained for use.
[0036] 3. Add a certain amount of 20% piperidine (piperidine / DMF=1:4, v:v) into the reactor, and shake it on a decolorizing shaker for 20 minutes to remove the Fmoc protecting group on the resin. After deprotection, wash four times with 3 times the resin volume of DMF, and then drain.
[0037] 4. Take a small amount of resin and test it with ninhydrin (Ninhydrin Nine Wells) method (two drops each of test A and test B, react at 100°C for 1 min), if the resin has color, it means that the deprotection is successf...
Embodiment 2
[0074] The immune activity experiment of embodiment 2 bioactive peptides
[0075] 1. Experiment on the ability of bioactive peptide SGSAAWDDSAGGAGGQGLRVTAL to promote macrophage phagocytosis of neutral red
[0076] 1. Experimental reagents and instruments:
[0077] Reagent: experimental animal balb / c mouse (male 6-8 weeks old) Animal Experiment Center, College of Agriculture and Biology, Shanghai Jiaotong University; mouse spleen lymphocyte-derived bioactive peptide SGSAAWDDSAGGAGGQGLRVTAL obtained in Example 1; LPS, purchased from Sigma ; Neutral red staining solution, produced by Biyuntian Institute of Biotechnology.
[0078] Equipment: LRH-250F biochemical incubator Shanghai Heng Technology Co., Ltd.; GL-22M high-speed refrigerated centrifuge Shanghai Luxiangyi Centrifuge Instrument Co., Ltd.; Hera cell 150 CO2 Incubator Heraeus; Dragon WellscanMK3 microplate reader Labsystems.
[0079] 2. Experimental method:
[0080] The number of cells added is 2×10 6 100 μl / well of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| ion source temperature | aaaaa | aaaaa |
| collision energy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com