Automatic virus treatment method
A treatment method, virus technology, applied in biochemical equipment and methods, biomass post-treatment, biomass pre-treatment, etc., can solve the problems of increased cost and detection time, time-consuming, complicated process, etc., and achieve accurate and reliable detection results , The effect of reasonable processing flow configuration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
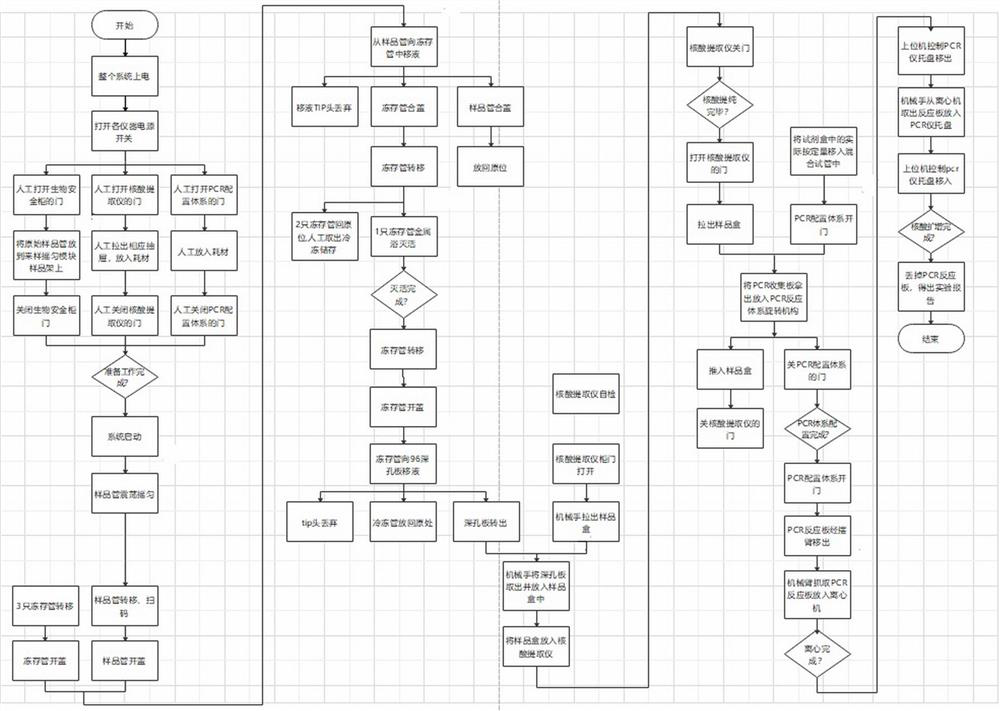
[0032] The specific implementation of the automatic virus processing method of the present invention will be described in detail below in conjunction with the accompanying drawings.
[0033] See attached figure 1 , the virus automatic processing method is realized by using a virus automatic processing system, and the virus automatic processing system includes a biological safety cabinet, a virus pretreatment unit, a nucleic acid purifier, a system construction unit, a PCR instrument and a control unit, and each unit and equipment complete each stage-specific features. The biological safety cabinet is used for the safety guarantee function of virus pretreatment. The virus pretreatment unit completes sample separation, freezing and inactivation, the nucleic acid purifier performs nucleic acid purification, and the body system construction unit performs mixed liquid treatment on the purified sample, and then The PCR instrument performs amplification and completes the detection. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com