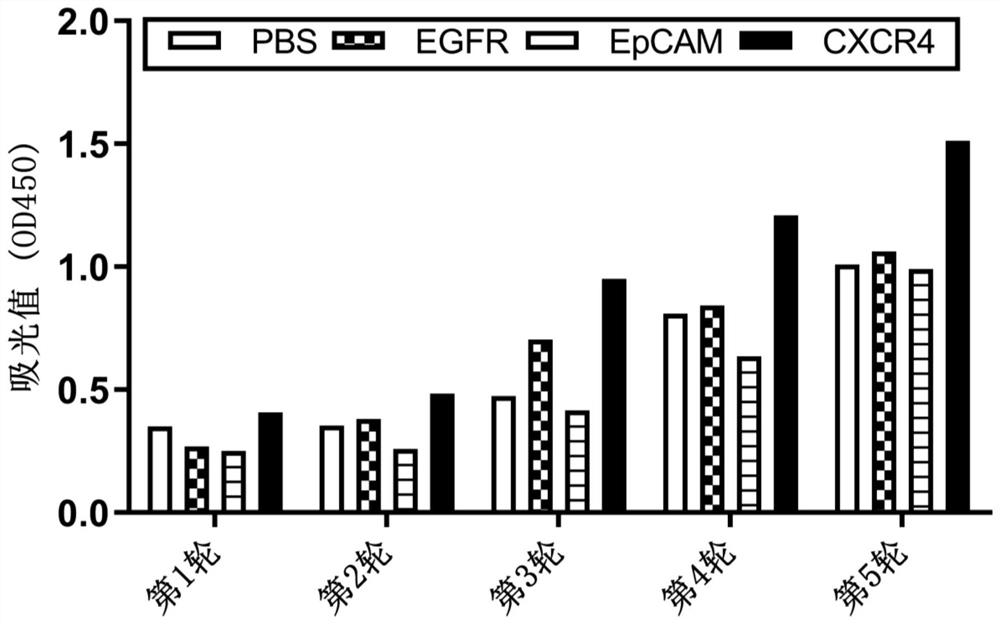
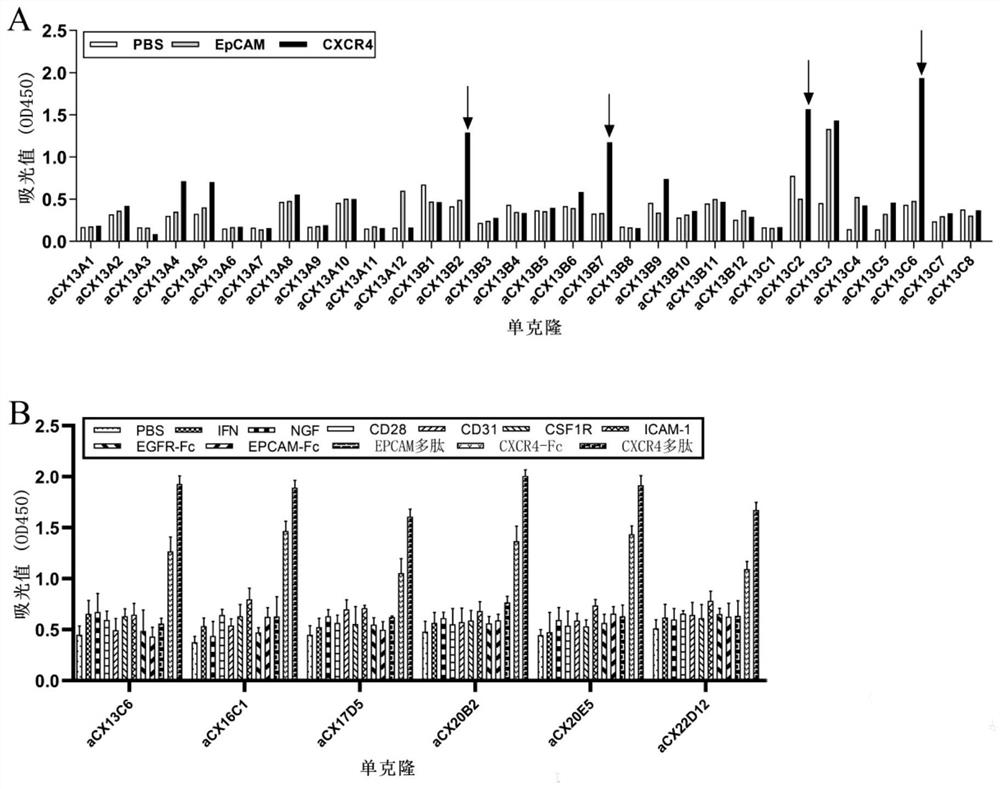
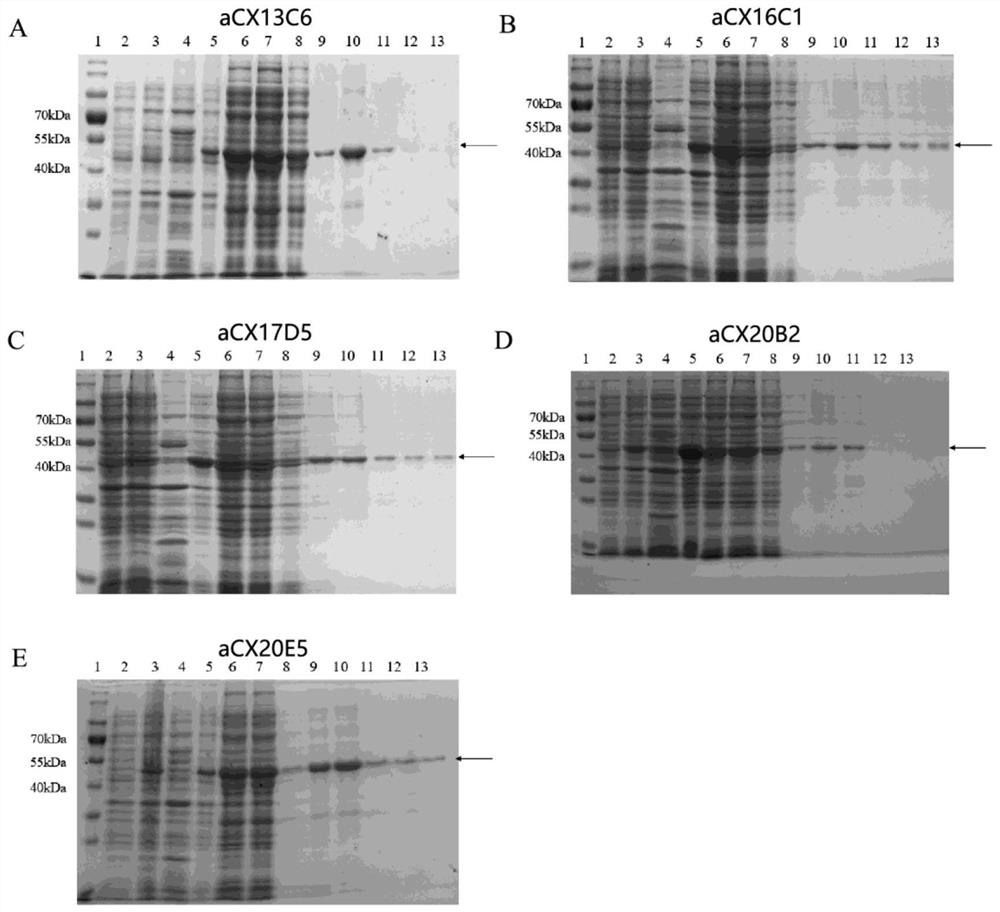
Affinity maturation binding protein bound with CXCR4 and application
A technology of binding protein and affinity, applied in the field of binding protein, can solve problems such as toxic side effects and cell damage, and achieve the effect of promoting application and reducing production cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064]Example 1 Construction of phage affinity maturation binding protein library
[0065] (1) Using the wild-type binding protein aCX82 as a template (the amino acid sequence encoding the aCX82 binding protein is shown in SEQ ID NO: 26, and the nucleotide sequence encoding the aCX82 binding protein is shown in SEQ ID NO: 34), according to GeneMorph IIRandom Mutagenesis The instruction manual of Kit carries out error-prone PCR, amplifies the target gene by the method of error-prone PCR, and primers are Error-F2 (nucleotide sequence as shown in SEQ ID NO:37) and Error-R2 (nucleotide sequence as shown in SEQ ID NO:37) NO:38).
[0066] (2) After the PCR reaction, use agarose gel electrophoresis to identify error-prone PCR products.
[0067] (3) Perform gel recovery of error-prone PCR products according to the instructions of the EZNA Gel Extraction Kit.
[0068] (4) The pComb3XSS phage display plasmid and the error-prone PCR product were subjected to a double enzyme digestion r...
Embodiment 2
[0074] Example 2 preparation of helper phage
[0075] (1) Line the TG1 Escherichia coli clone strain in three zones on a petri dish with TYE solid medium, and place the marked petri dish upside down in a 37°C incubator for about 14 hours.
[0076] (2) Pick a single colony of TG1 on the petri dish and inoculate it into 5 mL of 2×TY liquid medium, and culture overnight at 37°C and 250 rpm.
[0077] (3) Transfer the bacterial solution obtained in step (2) to another 5mL 2×TY liquid medium at a ratio of 1:100 by volume, and culture it at 37°C and 250rpm until the bacterial solution OD 600nm About 0.5.
[0078] (4) The KM13 helper phage was serially diluted with PBS (10 12 pfu / mL~10 4 pfu / mL).
[0079] (5) Take 200 μL of the bacterial solution cultured in step (3), add 10 μL of the diluted KM13 helper phage, and place the mixture in a 37° C. water bath for 30 minutes to obtain mixture A.
[0080] (6) Mix low-melting point agarose and 2×TY liquid medium, heat until completely m...
Embodiment 3
[0085] Example 3 Amplification of Phage Affinity Maturation Binding Protein Library
[0086] (1) Thaw the bacterial library containing affinity-matured binding protein particles on ice, add 100 μL to 50 ml 2×TY medium (containing 100 μg / mL ampicillin+ and 1% w / v glucose), and shake at 37 ° C and 220 rpm for 2 h , until OD 600nm = 0.5.
[0087] (2) Then add 5×10 11 The pfu KM13 helper phage was placed in a constant temperature water bath at 37°C for 30 minutes. Centrifuge in a small high-speed centrifuge at 3200g for 10min. The pellet was gently resuspended in 2×TY liquid medium (containing 100 μg / mL Amp+ and 50 μg / mL Kana+), and cultured with shaking at 25° C. and 220 rpm for 20 h.
[0088] (3) Centrifuge in a small high-speed centrifuge, centrifuge at 3200g for 20min, retain the supernatant solution, absorb 40ml of the supernatant solution, add 10ml of 20% w / v PEG-NaCl solution, mix gently, and place in ice bath for 2h.
[0089] (4) Centrifuge in a small high-speed centr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Protein molecular weight | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com