High-temperature-resistant sintering catalyst for preparing chlorine by oxidizing hydrogen chloride and preparation method of high-temperature-resistant sintering catalyst
A technology of oxidation and high temperature resistance, applied in the direction of metal/metal oxide/metal hydroxide catalyst, preparation with chloride, physical/chemical process catalyst, etc., to achieve the effect of enhancing effect and improving high temperature sintering performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
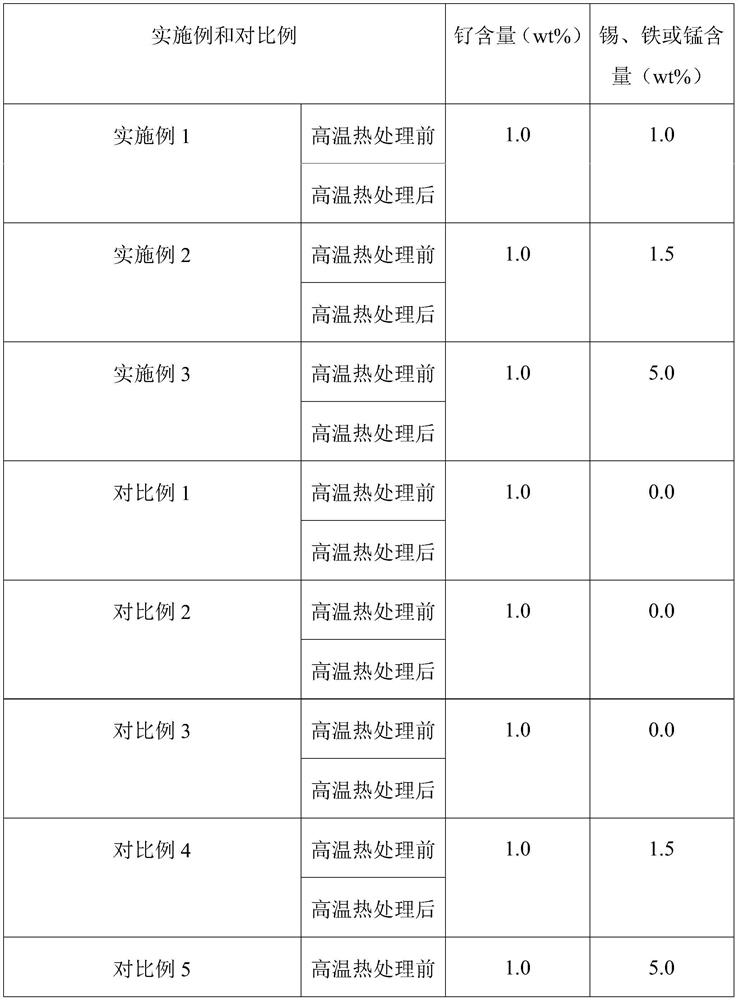
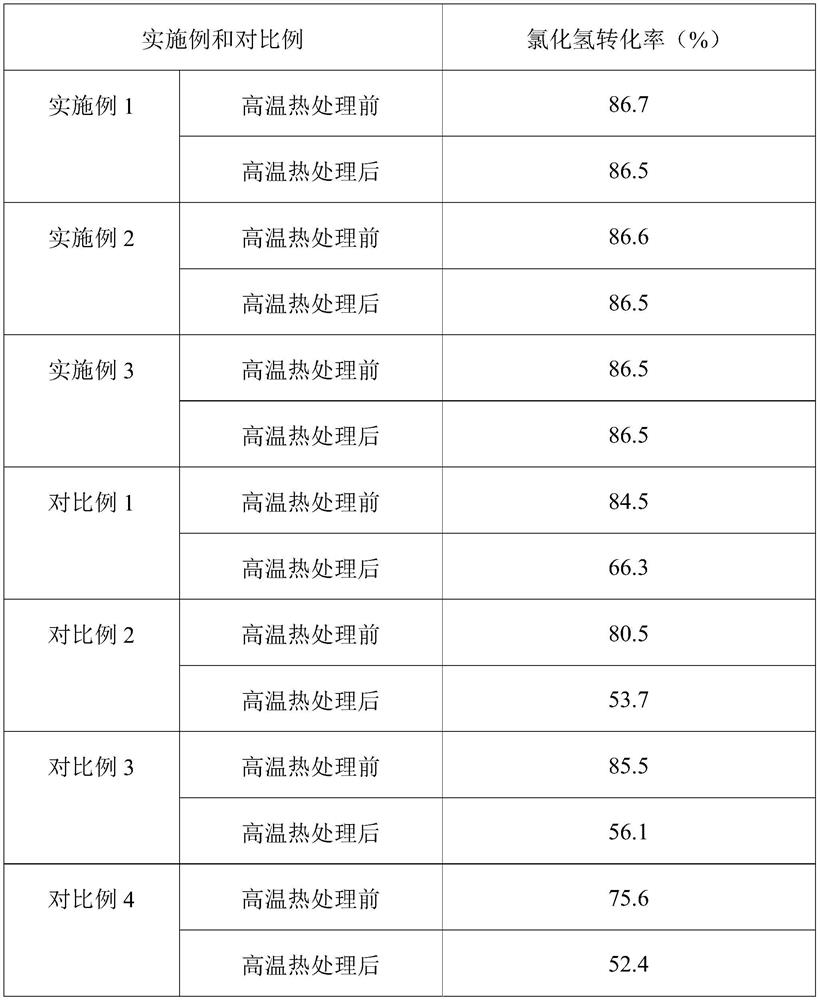
Embodiment 1
[0022] (1) impregnating powdery titanium dioxide and tin tetrachloride aqueous solution in equal volumes, drying and roasting to obtain a tin-doped titanium dioxide carrier, the powdery titanium dioxide is in the rutile crystal form, and the concentration of the tin tetrachloride aqueous solution is 0.1 g / mL, the drying condition is drying at 100°C for 6 hours, and the roasting condition is roasting at 500°C for 6 hours in an oxygen atmosphere;
[0023] (2) impregnate the tin-doped titanium dioxide carrier obtained in step (1) with an aqueous solution of ruthenium trichloride to obtain a catalyst through drying and roasting, the concentration of the aqueous solution of ruthenium trichloride is 0.1 g / mL, and the drying The condition is drying at 75° C. for 12 hours, and the calcination condition is sintering at 250° C. in an air atmosphere for 6 hours.
Embodiment 2
[0025] (1) impregnating equal volumes of powdery titanium dioxide and tin tetrachloride aqueous solution, drying and roasting to obtain a tin-doped titanium dioxide carrier, the powdery titanium dioxide is a mixed crystal form of rutile crystal form and anatase crystal form, so The weight percentage of the titanium dioxide of the rutile crystal form in the titanium dioxide of the mixed crystal form is 80%, the concentration of the tin tetrachloride aqueous solution is 0.15g / mL, the drying condition is 6 hours at 200°C, and the roasting condition Baking under an oxygen atmosphere at 350°C for 12 hours;
[0026] (2) impregnate the tin-doped titanium dioxide carrier obtained in step (1) with an aqueous solution of ruthenium trichloride to obtain a catalyst through drying and roasting, the concentration of the aqueous solution of ruthenium trichloride is 0.1 g / mL, and the drying The condition is drying at 60° C. for 6 hours, and the calcination condition is sintering at 250° C. in...
Embodiment 3
[0028] (1) The powdery titanium dioxide and the aqueous solution of tin tetrachloride are impregnated in equal volumes, dried and roasted to obtain a tin-doped titanium dioxide carrier, the powdery titanium dioxide is in the rutile crystal form, and the concentration of the aqueous solution of tin tetrachloride is 0.5 g / mL, the drying condition is drying at 100°C for 24 hours, and the roasting condition is roasting at 800°C for 4 hours in an oxygen atmosphere;
[0029] (2) impregnate the tin-doped titanium dioxide carrier obtained in step (1) with an aqueous solution of ruthenium trichloride to obtain a catalyst through drying and roasting, the concentration of the aqueous solution of ruthenium trichloride is 0.1 g / mL, and the drying The condition is drying at 400° C. for 18 hours, and the calcination condition is 12 hours at 250° C. in an air atmosphere.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com