Method for detecting histone acetyltransferase based on nano-enzyme
A technology of acetyltransferase and histone, which is applied in the field of nano-biological analysis and detection, can solve the problems that the application of histone acetyltransferase has not been reported, and achieve the effect of low cost, fast response and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
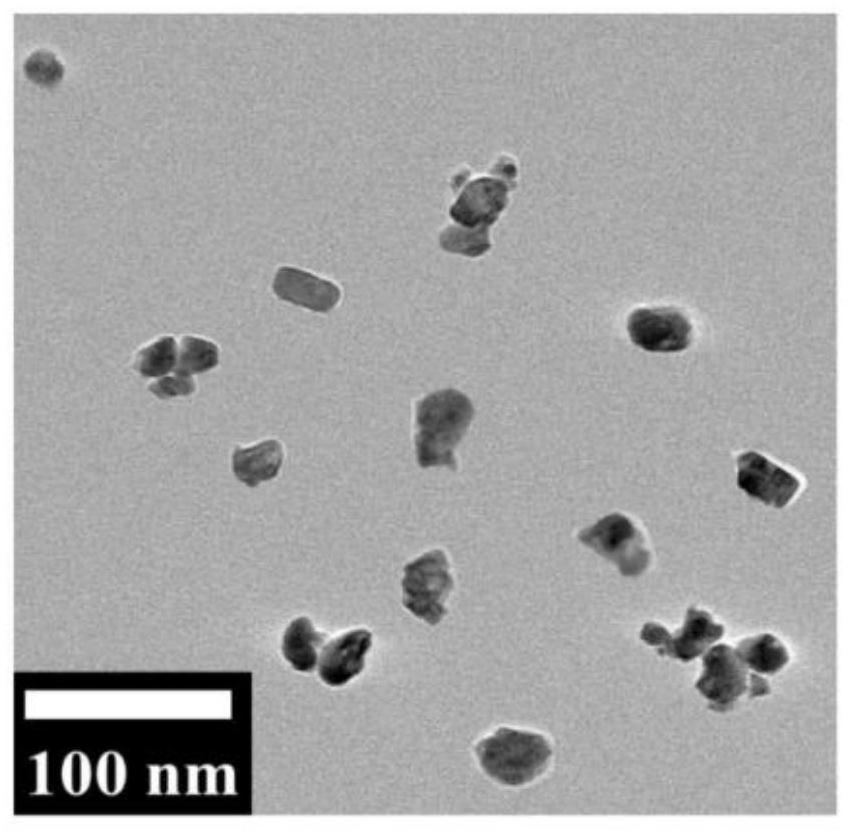
[0037] a. CuBi 2 o 4 Synthesis of nanomaterials: 1.0mmol Bi(NO 3 ) 3 ·5H 2 O was dispersed in 20mL of aqueous solution containing 0.7mL of concentrated nitric acid, stirred for 2h until the solution was clear and transparent; 10mL of Cu(NO 3 ) 2 ·3H 2 O (0.05mol / L) aqueous solution was added to the above clear solution; then 15mL NaOH aqueous solution (1.0mol / L) was added dropwise under continuous stirring until the solution turned into a blue-green precipitate. The final mixture was transferred to a Teflon-lined stainless steel autoclave and heated at 140 °C for 14 h. The product was alternately washed six times with ethanol and water, and dried under vacuum at 60°C overnight, and the final product was brown powder;
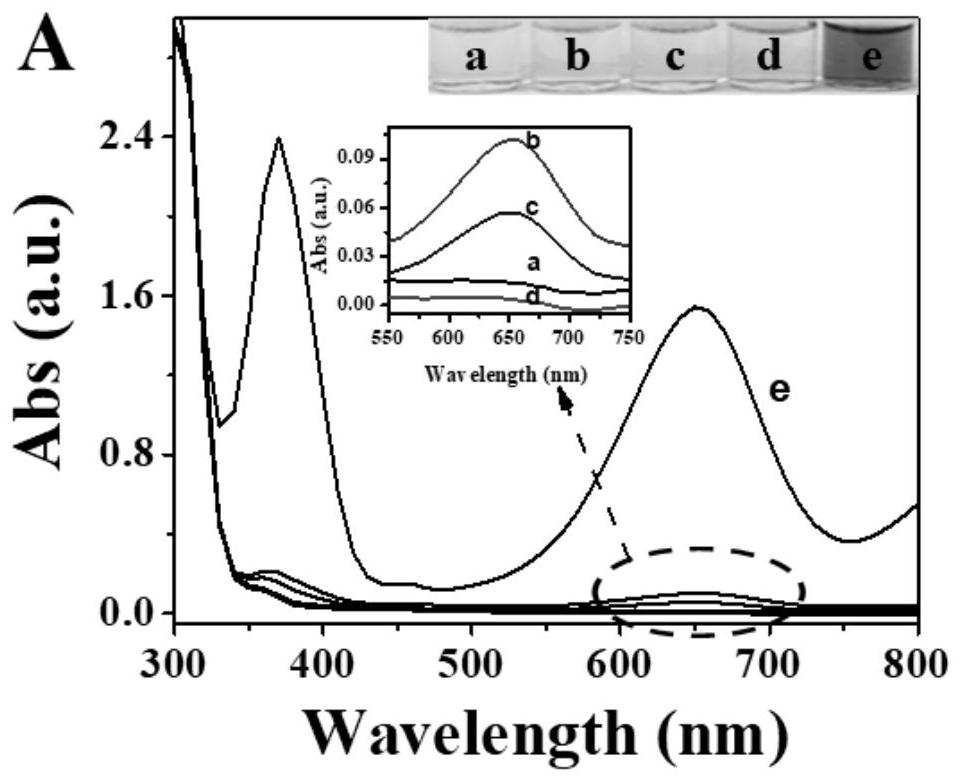
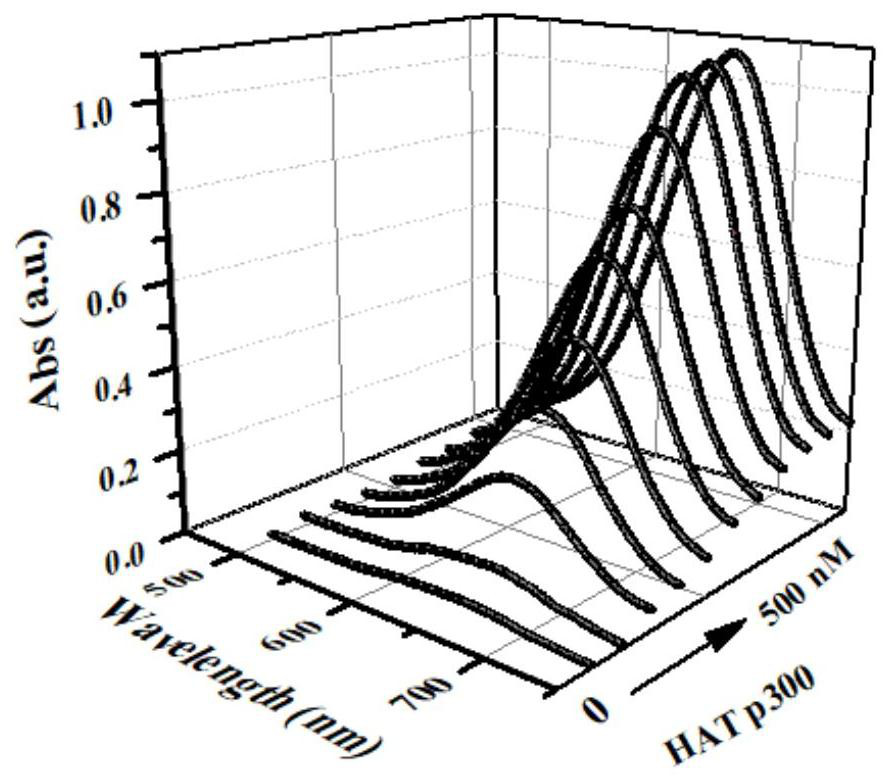
[0038] b. Determination of HAT p300: 20 μL of different concentrations of HAT p300 (0, 0.3, 1.0, 3.0, 10, 30, 100, 150, 200, 300, 500 μmol / L), 20 μL of 1.0 mmol / L acetyl-CoA and 20 μL of 1.0 mmol / L substrate peptide (RGKGGKGLGKGGAKA) were mixed and incub...
Embodiment 2
[0042] a. CuBi 2 o 4 Synthesis of nanomaterials: 1.0mmol BiCl 3 Disperse in 20mL aqueous solution containing 0.7mL concentrated nitric acid, stir for 2h until the solution is clear and transparent; 4 ) 2 ·5H 2 O (0.05mol / L) aqueous solution was added to the above clear solution; then 15mL NaOH aqueous solution (1.0mol / L) was added dropwise under continuous stirring until the solution turned into a blue-green precipitate. The final mixture was transferred to a Teflon-lined stainless steel autoclave and heated at 160 °C for 16 h. The product was alternately washed six times with ethanol and water, and dried under vacuum at 60°C overnight, and the final product was brown powder;
[0043] b. Determination of HAT p300: 20 μL of different concentrations of HAT p300 (0, 0.3, 1.0, 3.0, 10, 30, 100, 150, 200, 300, 500 μmol / L), 20 μL of 1.0 mmol / L acetyl-CoA and 20 μL of 1.0 mmol / L substrate peptide (RGKGGKGLGKGGAKA) were mixed and incubated at 30°C for 80min. After the incubatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com