Preparation method of vinorelbine tartrate
A technology of vinorelbine tartrate and vinorelbine, which is applied in the field of preparation of vinorelbine tartrate, can solve the problems of low purity of vinorelbine, difficulty in industrial production, and great difficulty in purification, and achieve simplified operation, simple operation, and reduced production cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] A preparation method of vinorelbine tartrate, comprising:
[0046] 1) Preparation steps of dehydrated vinblastine: using vinblastine sulfate and pendolin as starting materials, catalyzed by ferric chloride and reduced by sodium borohydride, to prepare dehydrated vinblastine;
[0047] 2) The preparation steps of bromoanhydrovinblastine: dehydrovinblastine and N-bromosuccinimide (NBS) carry out bromination reaction to obtain the bromoanhydrovinblastine crude product; the bromoanhydrovinblastine crude product is purified through a column to improve Its purity, obtains bromoanhydrovinblastine;
[0048] 3) The preparation steps of vinorelbine: debromination and rearrangement of bromoanhydrovinblastine and silver tetrafluoroborate aqueous solution to obtain crude product of vinorelbine; recrystallization of crude product of vinorelbine to obtain pure product of vinorelbine;
[0049] 4) Preparation steps of vinorelbine tartrate: the pure product of vinorelbine is reacted with...
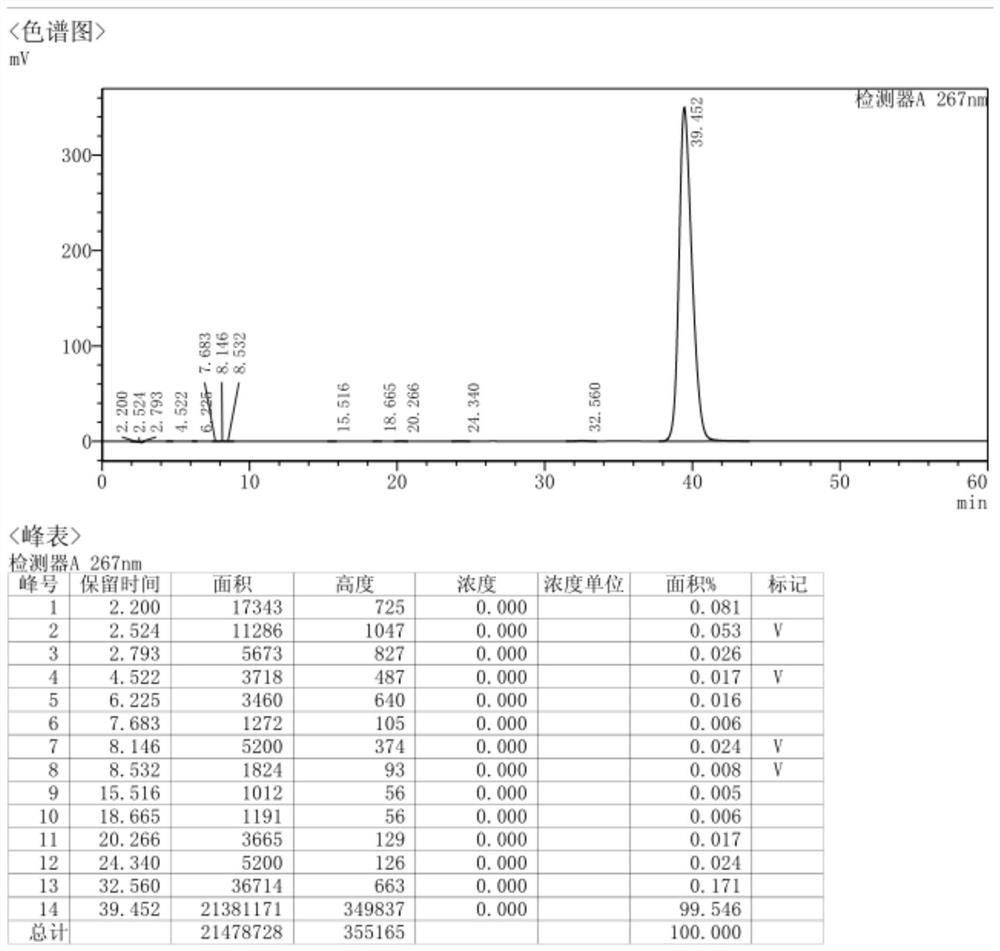
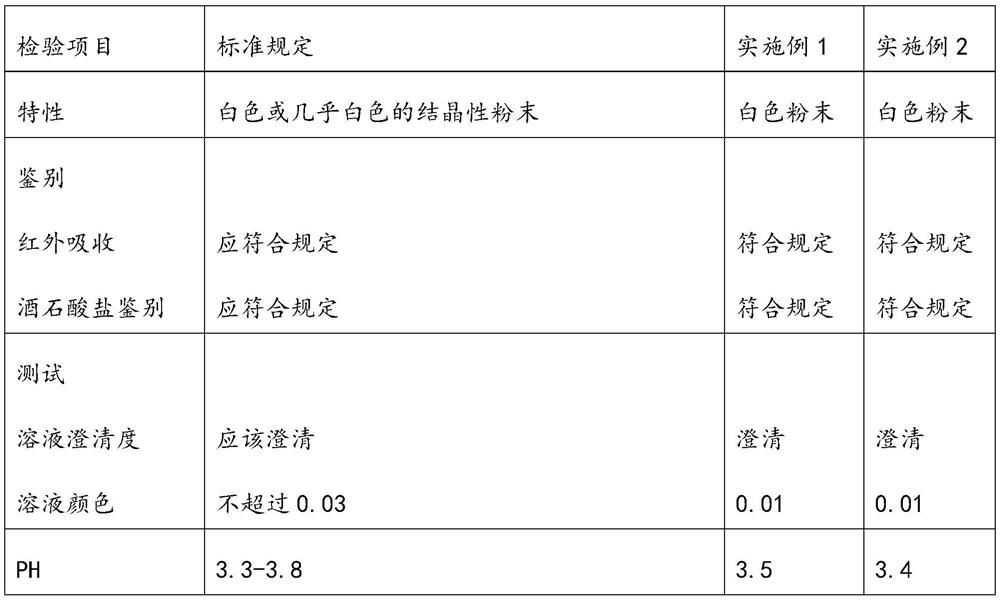
Embodiment 1
[0079] A preparation method of vinorelbine tartrate, comprising:
[0080] 1) The preparation steps of dehydrovinblastine: the specific preparation process is as follows:
[0081] 1-1) Preparation steps of liquid A: Take 4.4g of ventolin and 4g of vinblastine sulfate, add 40ml of tetrahydrofuran, add 20ml of hydrochloric acid solution, heat slightly, and dissolve under the condition of 35°C-40°C.
[0082] 1-2) Preparation steps of liquid B: Take 17g of ferric chloride, add 400ml of slightly heated purified water, add 10ml of hydrochloric acid solution, and use nitrogen to drive oxygen for about 10 minutes.
[0083] 1-3) Preparation steps of liquid C: Take 25g of ammonium chloride, add 80ml of slightly heated purified water to dissolve, add nitrogen to drive oxygen for about 10 minutes.
[0084] 1-4) Preparation steps of liquid D: Weigh about 1.2g of sodium borohydride and dissolve it in 70ml of dilute ammonia water. Dilute ammonia water refers to the mixed aqueous solution of...
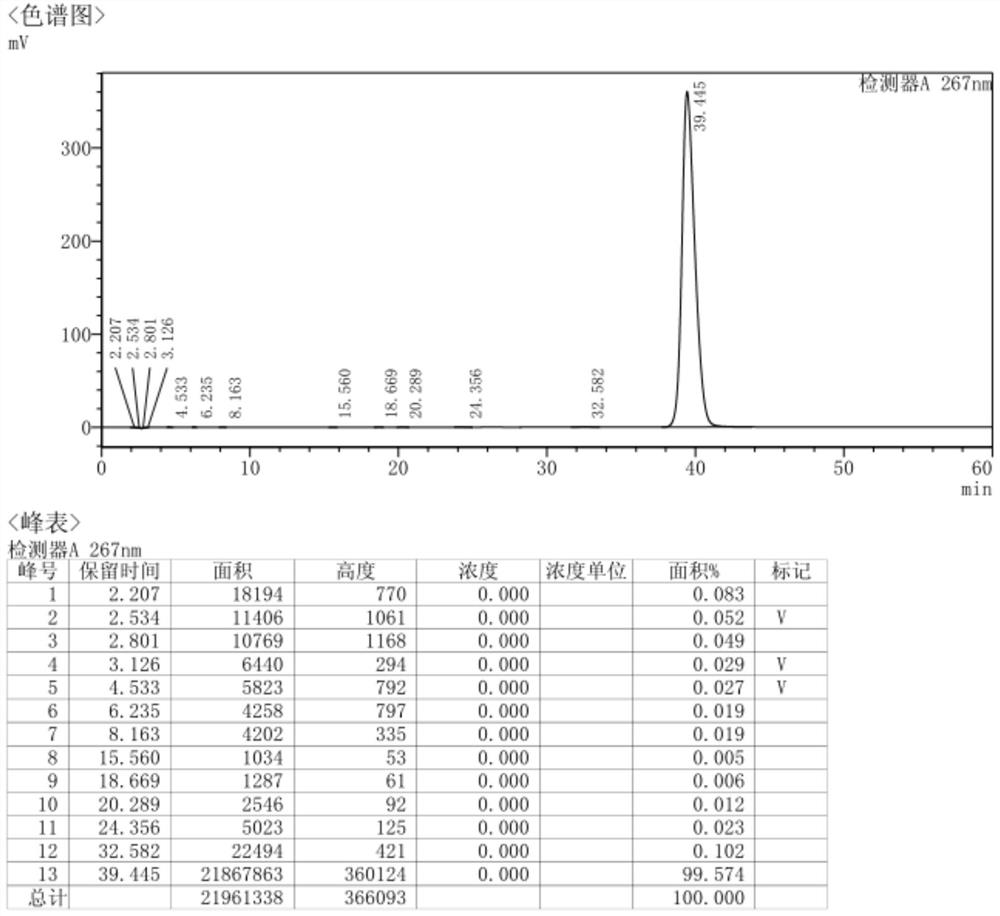
Embodiment 2
[0105] A preparation method of vinorelbine tartrate, comprising:
[0106] 1) The preparation steps of dehydrovinblastine: the specific preparation process is as follows:
[0107] 1-1) Preparation steps of liquid A: Take 4.4g of ventolin and 4g of vinblastine sulfate, add 40ml of trifluoroethanol, and add 20ml of hydrochloric acid solution to slightly heat (35°C-40°C) to dissolve.
[0108] 1-2) Preparation steps of liquid B: Take 17g of ferric chloride, add 400ml of slightly heated purified water, add 10ml of hydrochloric acid solution, and use nitrogen to drive oxygen for about 10 minutes.
[0109] 1-3) Preparation steps of liquid C: Take 25g of ammonium chloride, add 80ml of slightly heated purified water to dissolve, add nitrogen to drive oxygen for about 10 minutes.
[0110] 1-4) Preparation steps of liquid D: Weigh about 1.3g of sodium borohydride and dissolve it with 70ml of dilute ammonia water. Dilute ammonia water refers to the mixed aqueous solution of ammonia water w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com