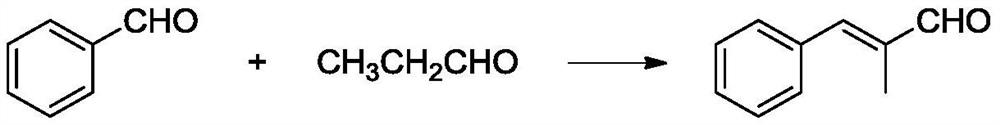
Method for synthesizing alpha-methylcinnamaldehyde from phenylpropionaldehyde
A technology of methyl cinnamaldehyde and phenylpropionaldehyde, applied in chemical instruments and methods, preparation of organic compounds, preparation of carbon-based compounds, etc., can solve the problems of poor economic benefits, average yield, and high cost of phase transfer catalysts, etc. Achieve the effects of simple operation, improved selectivity, and good industrial application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] At room temperature, two 500mL autoclaves (effective volume: 430mL) were sealed and filled with 0.5MPa nitrogen gas to keep the pressure for 30min. The pressure in the autoclave did not decrease, which proved that the sealing was good. Fill and discharge nitrogen 3 times, 0.3MPa each time, and finally reduce the inside of the kettle to normal pressure. Turn on the external heat preservation of the autoclave, and when the internal temperature of the first kettle reaches 30°C and the internal temperature of the second kettle rises to 40°C, start to continuously feed phenylpropanal (2.85mL / min) and aqueous formaldehyde ( 37%, 1.8mL / min) and sodium hydroxide aqueous solution (10%, 0.16mL / min), the feed molar ratio is phenylpropionaldehyde: formaldehyde: sodium hydroxide = 100:110:2; while feeding, open 2 For the stirring of the two reactors, the two reactors all use turbine-type stirring paddles with a rotation speed of 600rpm to promote the mass transfer of water and oil t...
Embodiment 2
[0040]At room temperature, two 500mL autoclaves (effective volume: 430mL) were sealed and filled with 0.5MPa nitrogen gas to keep the pressure for 30min. The pressure in the autoclave did not decrease, which proved that the sealing was good. Fill and discharge nitrogen 3 times, 0.3MPa each time, and finally reduce the inside of the kettle to normal pressure. Turn on the external heat preservation of the autoclave, and when the internal temperature of the first kettle reaches 30°C and the internal temperature of the second kettle rises to 60°C, start to continuously feed phenylpropanal (5.49mL / min) and aqueous formaldehyde ( 37%, 3.75mL / min) and sodium hydroxide aqueous solution (20%, 0.13mL / min), the feed molar ratio is phenylpropionaldehyde: formaldehyde: sodium hydroxide = 100:120:2; while feeding, open 2 For the stirring of the two reactors, the two reactors all use turbine-type stirring paddles with a rotation speed of 600rpm to promote the mass transfer of water and oil t...
Embodiment 3
[0042] At room temperature, two 500mL autoclaves (effective volume: 430mL) were sealed and filled with 0.5MPa nitrogen gas to keep the pressure for 30min. The pressure in the autoclave did not decrease, which proved that the sealing was good. Fill and discharge nitrogen 3 times, 0.3MPa each time, and finally reduce the inside of the kettle to normal pressure. Turn on the external heat preservation of the autoclave, and when the internal temperature of the first kettle reaches 30°C and the internal temperature of the second kettle rises to 30°C, start to continuously feed phenylpropanal (2.06mL / min) and aqueous formaldehyde ( 37%, 1.4mL / min) and sodium hydroxide aqueous solution (5%, 0.12mL / min), the feed molar ratio is phenylpropionaldehyde: formaldehyde: sodium hydroxide = 100:120:1; while feeding, open 2 For the stirring of the two reactors, the two reactors all use turbine-type stirring paddles with a rotation speed of 600rpm to promote the mass transfer of water and oil tw...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com