A kind of new synthetic method of pymetrozine intermediate nicotinic aldehyde
A synthesis method and pymetrozine technology are applied in the field of synthesis technology of pesticide intermediates, can solve the problem that the synthesis technology of nicotinic aldehyde cannot be applied to large-scale production and the like, and achieve the effect of simple process steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
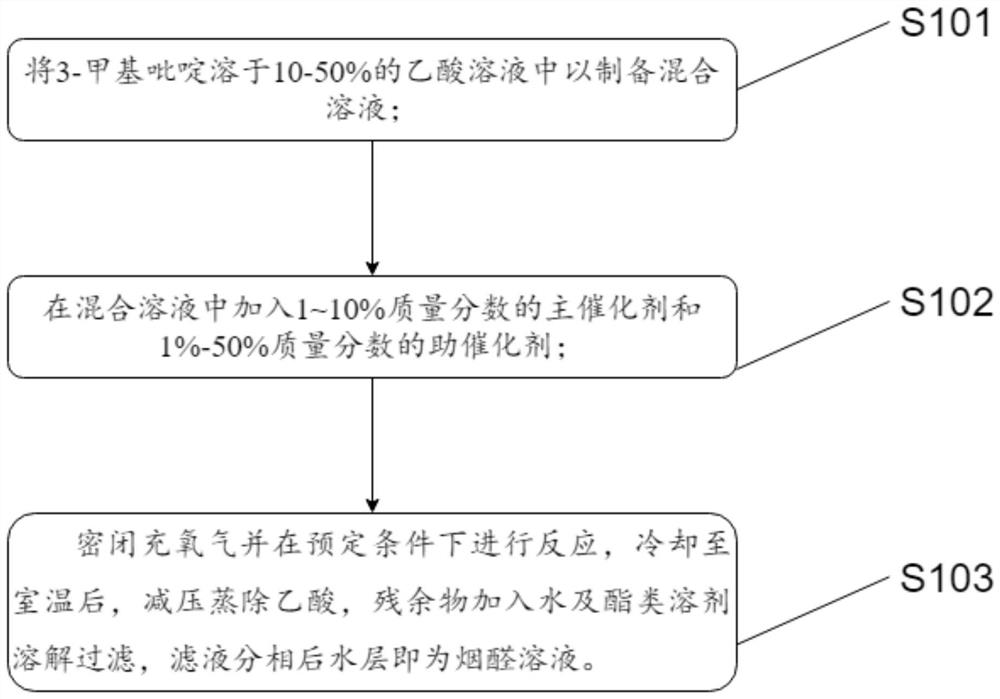
[0022] A new method for synthesizing pymetrozine intermediate nicotinaldehyde, comprising the steps of:
[0023] S101: making 10-50% acetic acid solution with 3-picoline;
[0024] S102: adding 1-10% by mass of the main catalyst and 1%-50% by mass of the co-catalyst into the solution;
[0025] S103: airtightly fill with oxygen and react under predetermined conditions. After cooling to room temperature, distill off acetic acid under reduced pressure, add water and ester solvent to dissolve the residue and filter.
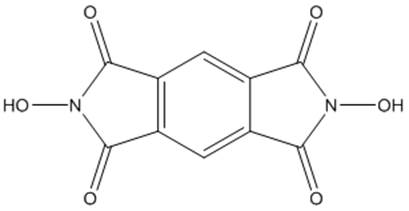
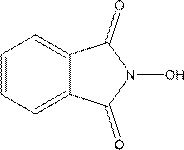
[0026] Further, the main catalyst is a nitrogen hydroxyl compound, including compound I, compound II and compound III.
[0027] Further, the molecular formula of the compound I is:
[0028]
[0029] Further, the molecular formula of the compound II is:
[0030]
[0031] Further, the molecular formula of the compound III is:
[0032]
[0033] Further, the predetermined conditions are: the oxygen pressure is 1-10 atm, and the reaction is at 20-100° C. for 2...
specific Embodiment 1
[0037] The 3-picoline of 93g (1mol) is dissolved in the acetic acid of 1L, is made into 10% acetic acid solution, it is added into the autoclave 10 of 2L, then in still, add catalyst (I) 25g (0.1mol, 10 % equivalent), 9 g of 68% concentrated nitric acid, sealed reaction kettle 10 and replaced the air with oxygen, under 1 atm oxygen pressure, raised the temperature to 100 ° C, kept the temperature for 2 hours, cooled down to room temperature, removed acetic acid under negative pressure, and poured into the residue Add 200ml of water and 100ml of ethyl acetate, stir for 0.5h and filter a small amount of black insoluble matter, the filtrate is layered, the upper layer is the product layer, the quantitative analysis yield is 70%, the organic layer is precipitated to recover raw materials and catalysts.
specific Embodiment 2~5
[0039] The nicotine aldehyde product was prepared according to the operation process of the specific example 1, the difference is that the type of main catalyst and co-catalyst used, temperature, pressure, etc. are shown in Table 1, and the results are shown in Table 1.
[0040]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com