Novel method for preparing ethambutol hydrochloride
A technology for ethambutol hydrochloride and ethambutol, which is applied in the field of preparing ethambutol hydrochloride, and can solve the problems of multiple by-products, excess, troublesome separation and purification, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1: the synthesis of L-2-aminobutyric acid
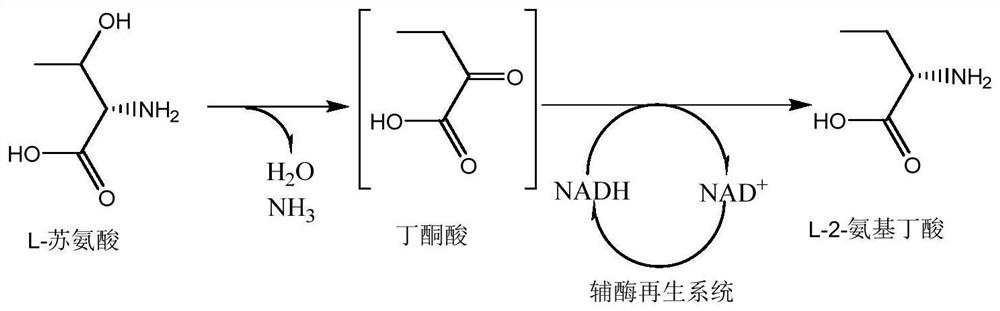
[0020] Add 2382.4g L-threonine (20mol), 1261.2g ammonium formate (1mol), 1L TD enzyme solution, 1L FDH / FDH co-expression crude enzyme solution, 2L NAD+ and The mixed solution of pyridoxal phosphate was reacted at 40°C for 24 hours. After the macroporous resin adsorbed macromolecular impurities, the product was concentrated, and ethanol was added to dissolve the impurities. The obtained solid product was filtered and dried for the next reaction. The dried product is 1959.2g of L-2-aminobutyric acid with a purity of 96%, and the yield is 91%.
Embodiment 2
[0021] Embodiment 2: the synthesis of diamide dicarboxylic acid
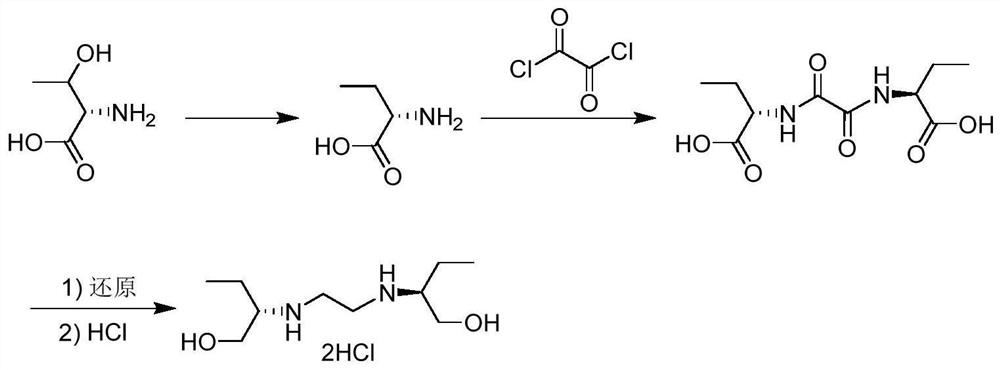
[0022] 200 milliliters of dichloromethane, 103.12 g of L-2-aminobutyric acid (1 mol), 76.16 g of oxalyl chloride (0.6 mol) and 200 milliliters of triethylamine were added to the reaction kettle, and at 100° C., the amidation reaction was carried out for 12 hours. Obtain (2S,2S')-N,N'-bis(2-butyric acid)-oxamide, that is, diamide dicarboxylic acid. After the reaction, the excess low-boiling point solvent was removed by rotary evaporation, and the remaining solid was recrystallized in an aqueous solution and dried to obtain 213.4 g of diamide dicarboxylic acid with a yield of 82%.
Embodiment 3
[0023] Embodiment 3: the synthesis of ethambutol
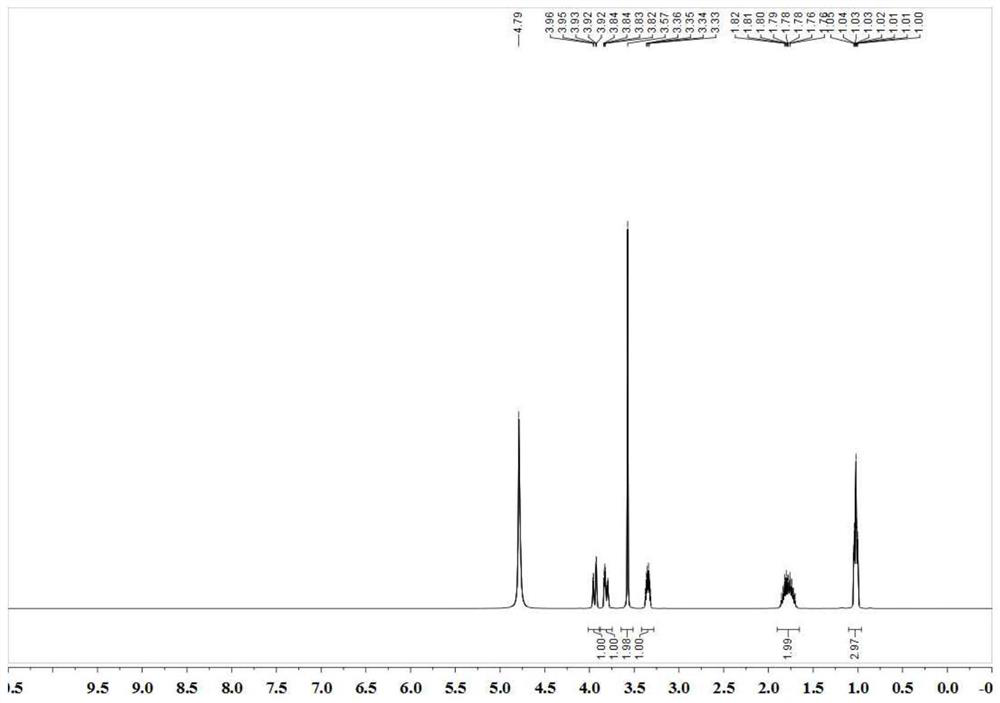
[0024] Add 1L methanol, 130.13g diamide dicarboxylic acid (0.5mol), catalyst 22.17g Co(BF4)2·6H2O (0.025mol) and equimolar amount of tridentate phosphine ligand 1,1,1-tri (Diphenylphosphinomethyl)ethane (15.62 g) was fed with hydrogen, the pressure was maintained at 0.5 MPa, and catalytic hydrogenation was carried out at 150° C. for 2 h to obtain 183.88 g of ethambutol with a yield of 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com