Synthesis method of 2-aldehyde thiochromone compound
A technology for the synthesis of aldehyde thiochromones and methods, which is applied in the field of synthesis of organic compounds, can solve the problems of unstable yield, cumbersome separation of by-products, and poor repeatability, and achieve simple post-processing operations and a wide range of substrates , good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
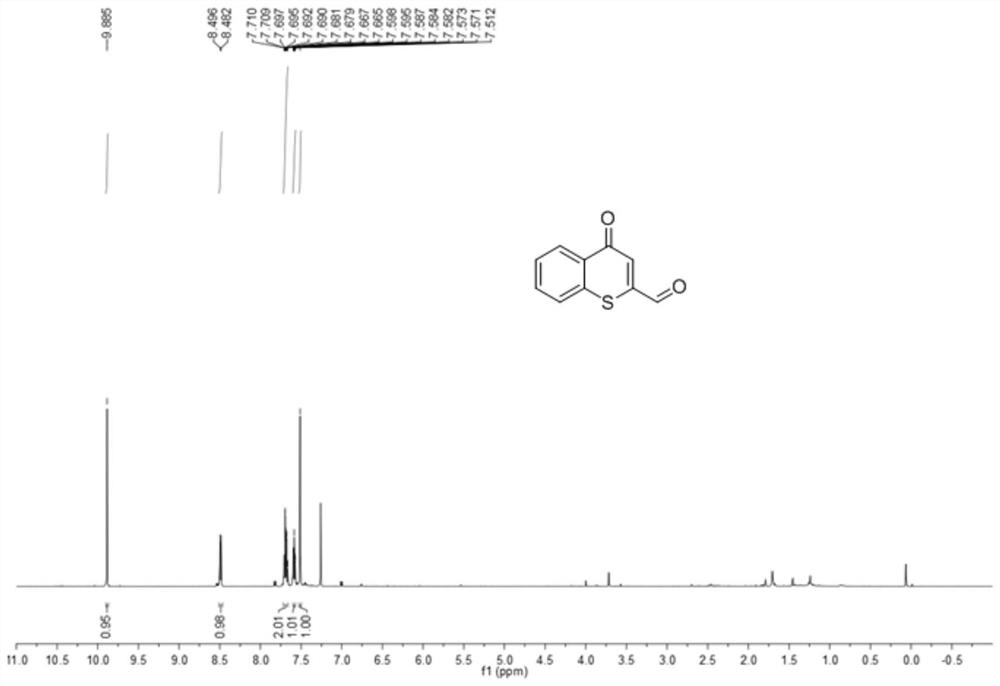
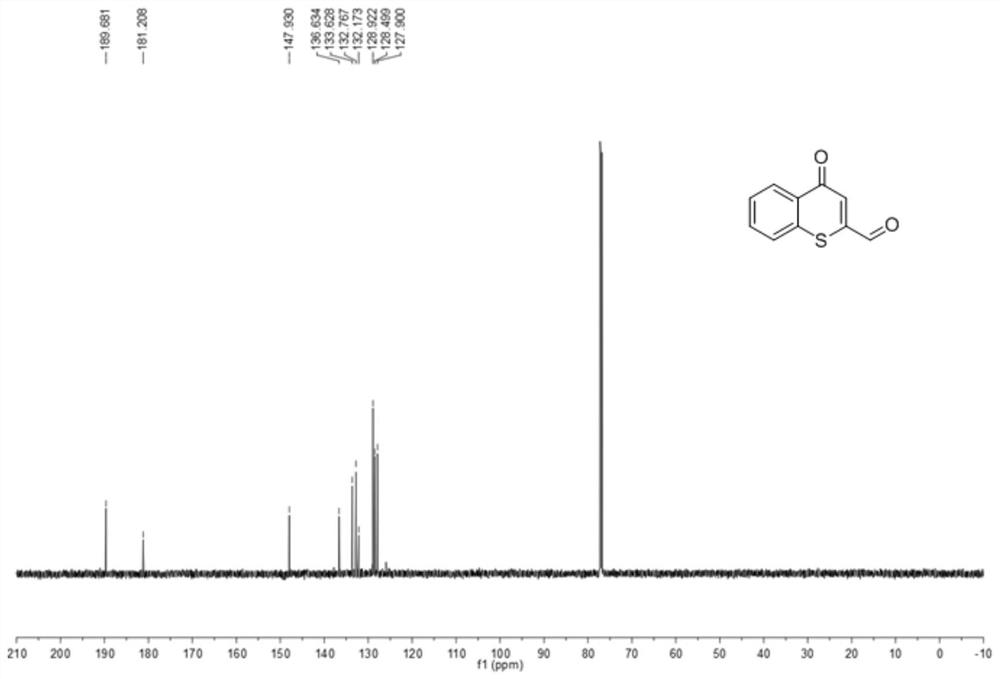
[0027] Preparation of 2-aldehydethiochromone
[0028]
[0029] 150mg (0.85mmol) of 2-methylthiochromone shown in formula I-1 was added to a 25mL round bottom flask, and 4.3mL of N,N-dimethylformamide was added to dissolve 2-methylthiochromone, and then Add 0.23mL (1.70mmol) N,N-dimethylformamide dimethyl acetal, reflux reaction at 150°C for 4h, cool down to room temperature after the reaction, add water to the reaction solution, and then add dichloromethane for separation and extraction Three times, the organic phases were combined, dried with anhydrous magnesium sulfate, suction filtered with a sand core funnel, and the solvent was evaporated under reduced pressure to obtain the enamine intermediate shown in formula II-1. Add the enamine intermediate shown in formula II-1 and 546mg (2.55mmol) sodium periodate into a 25mL round bottom flask, add 2.8mL tetrahydrofuran and 2.8mL water, and react at room temperature for 2h. Add saturated aqueous sodium thiosulfate solution to...
Embodiment 2
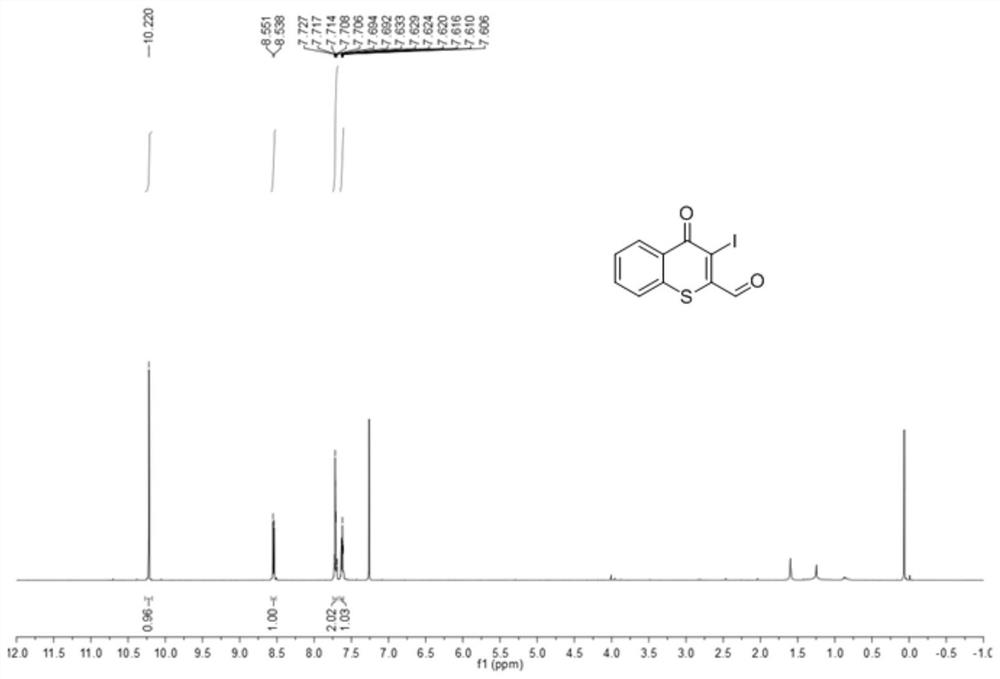
[0031] Preparation of 2-formyl-3-iodothiochromone
[0032]
[0033] Add 75mg (0.25mmol) of 2-methyl-3-iodothiochromone represented by formula I-2 into a 10mL round bottom flask, add 2.5mL N,N-dimethylformamide to dissolve 2-methyl- 3-iodothiochromone, then add 66 μL (0.5 mmol) N,N-dimethylformamide dimethyl acetal, 150 ° C reflux reaction for 4 hours, after the reaction is completed, cool down to room temperature, add water to the reaction solution, and then Dichloromethane was added for liquid separation and extraction three times, the organic phases were combined, dried over anhydrous magnesium sulfate, filtered through a sand core funnel, and the solvent was evaporated under reduced pressure to obtain the enamine intermediate of formula II-2. Add the enamine intermediate shown in formula II-2 and 159mg (0.74mmol) sodium periodate into a 10mL round-bottomed flask, add 1.3mL tetrahydrofuran and 1.3mL water, and react at room temperature for 2h. Add saturated aqueous sodiu...
Embodiment 3
[0035] Preparation of 2-aldehyde-3-phenylthiochromone
[0036]
[0037] Add 250 mg (0.98 mmol) of 2-methyl-3-phenylthiochromone shown in formula I-3 into a 25 mL round bottom flask, add 4.0 mL of N,N-dimethylformamide to dissolve 2-methyl-3- 3-Phenylthiochromone, then add 0.26mL (1.96mmol) N,N-dimethylformamide dimethyl acetal, reflux reaction at 150°C for 6h, cool down to room temperature after the reaction, add water to the reaction solution , and dichloromethane was added for liquid separation and extraction three times, the organic phases were combined, dried with anhydrous magnesium sulfate, suction filtered with a sand core funnel, and the solvent was evaporated under reduced pressure to obtain the enamine intermediate of formula II-3. Add the enamine intermediate shown in formula II-3 and 631mg (2.95mmol) sodium periodate into a 25mL round-bottomed flask, add 2.3mL tetrahydrofuran and 2.3mL water, and react at room temperature for 1 h. Add saturated aqueous sodium t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com