Benzocycloheptanopyridine compound, its preparation method and its use
A technology of benzocycloheptane and compounds, which is applied in the field of its preparation and benzocycloheppanopyridine compounds, can solve the problems of lack of reference substances, affecting the detection and monitoring of impurity compounds, and difficulty in separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
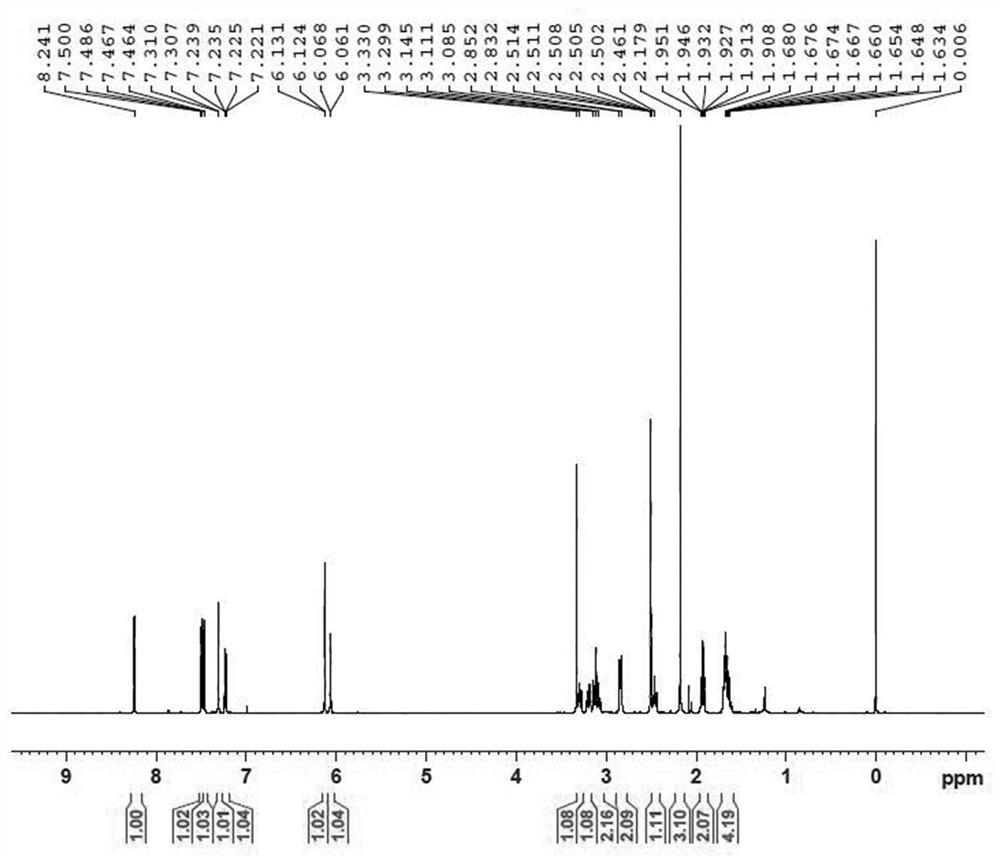
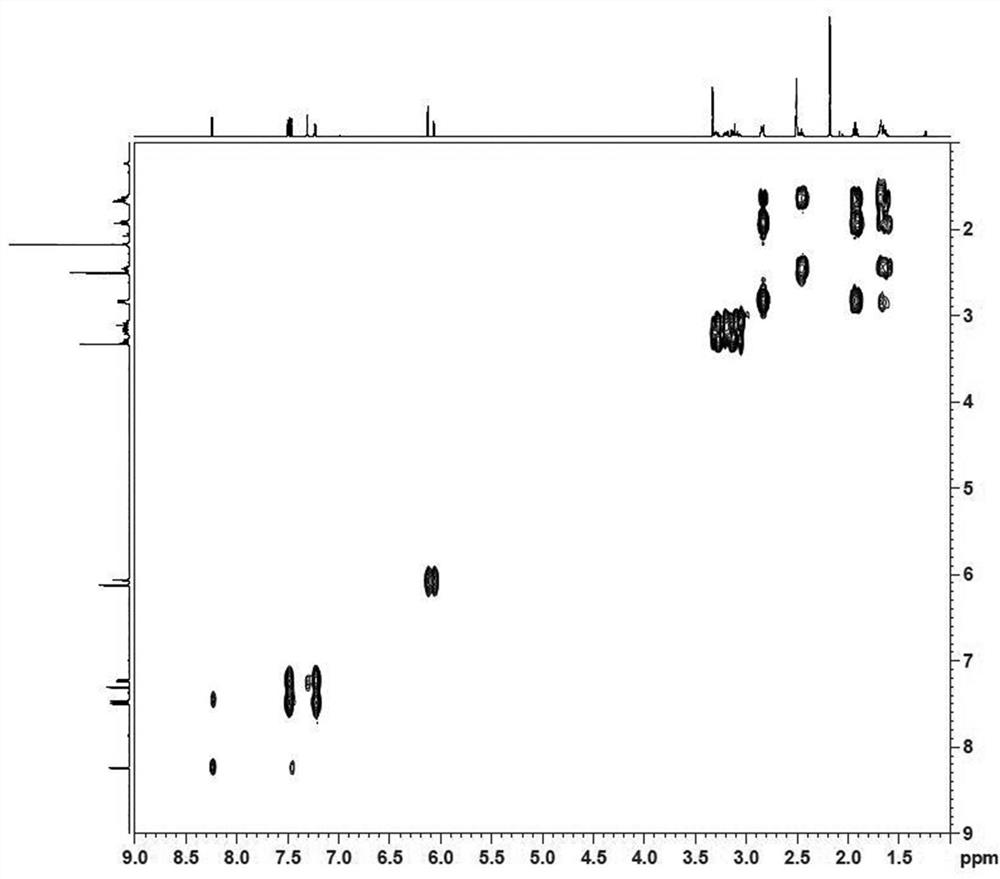
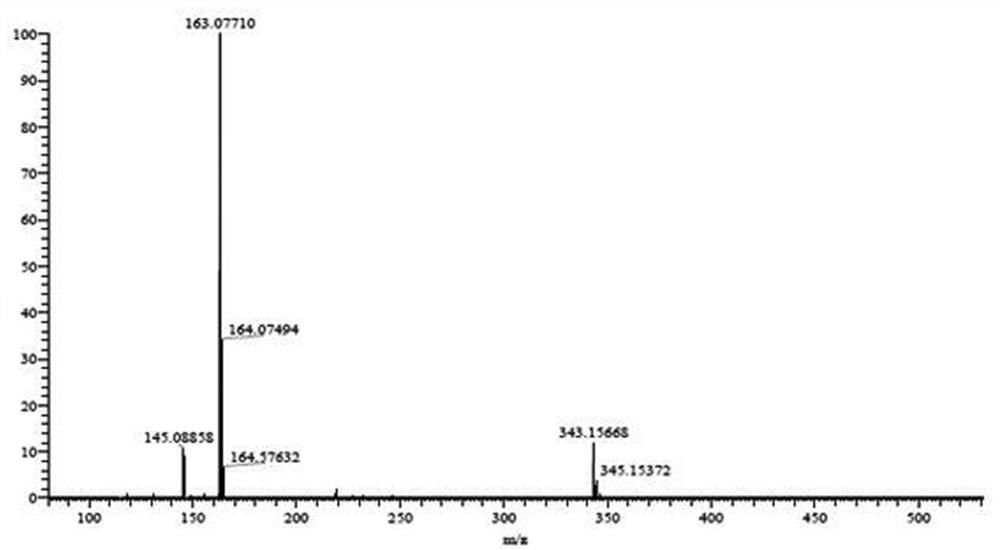
Image
Examples
preparation example Construction
[0046]In the first aspect, the embodiment of the present invention provides a method for preparing the benzocycloheptanopyridine compound as described in the first aspect, comprising the following steps:
[0047]
[0048] (1) Synthesis of format reagents:
[0049] Magnesium and N-methyl-4-chloropiperidine are heated and reacted in the first reaction solvent under the action of an initiator to obtain Grignard reagent;
[0050] (2) Synthesis of Compound I:
[0051] 8-chloro-5,6-dihydro-11H-benzo[5,6]cycloheptano[1,2-b]pyridin-11-one (Formula II) and the Grignard reagent in the second reaction solvent The reaction is carried out to obtain compound I, and the reaction temperature is 0°C~70°C;
[0052] (3) Synthesis of Compound IV:
[0053] Compound I is reacted in the third reaction solvent under the action of a reducing agent to obtain compound IV.
[0054] In the preparation method of the benzocycloheptanapyridine compound provided in the embodiment of the present inventi...
Embodiment 1
[0091] (1) Synthesis of format reagents:
[0092] Add magnesium chips (5.72g, 0.24mol) to 220mL tetrahydrofuran, start stirring, add 1,2-dibromoethane (6mL, 0.07mol), heat up to 65°C, add N-methyl-4-chloro Piperidine (32.01g, 0.24mol), after the reaction is initiated, react at 65°C until the magnesium chips disappear completely to obtain the Grignard reagent, cool down to room temperature, add N,N,N,N- to the Grignard reagent Tetramethylethylenediamine (27.90 g, 0.24 mol) was stirred evenly to obtain the first mixed solution.
[0093] (2) Synthesis of Compound I:
[0094] 8-Chloro-5,6-dihydro-11H-benzo[5,6]cyclohepta[1,2-b]pyridin-11-one (30.06 g, 0.12 mol) was added to 250 mL tetrahydrofuran, Stir and dissolve to obtain the second mixed solution, add the second mixed solution dropwise to the first mixed solution, control the reaction temperature to 10°C, and continue the reaction after the dropwise addition, the reaction time is 30min, the reaction After the end, add satur...
Embodiment 2
[0109] (1) Synthesis of Grignard Reagent: It is exactly the same as step (1) in Example 1.
[0110] (2) Synthesis of compound I: exactly the same as step (1) in Example 1.
[0111] (3) Synthesis of Compound IV:
[0112] Add 9.79g of 8-chloro-3-(1-methylpiperidin-4-yl)-5,6-dihydro-11H-benzo[5,6]cyclohepta[1, 2-b] Pyridin-11-one (formula Ⅰ, 0.03mol), 100mL methanol and 20mL dichloromethane, stir until the solution is clear, cool down to 15°C, control the reaction temperature to 15°C, add sodium borohydride (1.09g, 0.03mol) was added into the reaction solution in three batches, and the reaction time was 2 hours. After the reaction finishes, add 70mL water to the reaction solution, quench the reaction, extract with 70mL dichloromethane, the organic phase is in the lower floor, separate the layers, the organic phase obtained is dried with anhydrous sodium sulfate, remove sodium sulfate by filtration, and the filtrate is reduced Evaporate under pressure to obtain the crude produc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com