A kind of synthetic method of 5-amino-3-cyanopyridine carboxylic acid methyl ester hydrochloride
A technology of cyanopicolinic acid and methyl ester hydrochloride is applied in the field of synthesis of 5-amino-3-cyanopicolinate methyl ester hydrochloride, and achieves the effects of high yield, simple operation and short route.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
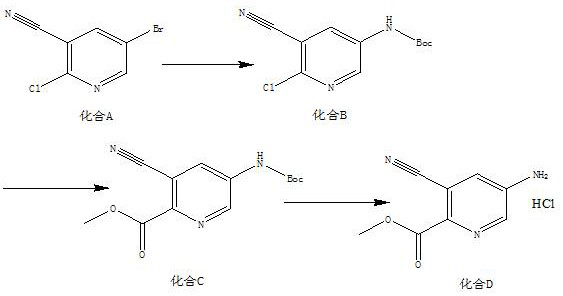
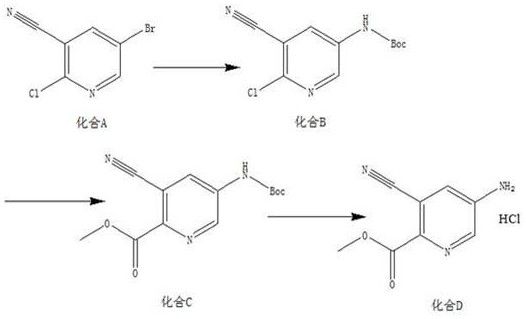
[0019] A kind of synthetic method of 5-amino-3-cyanopicolinate methyl ester hydrochloride, the method comprises the steps:
[0020] (1) According to compound A, tert-butyl carbamate, Pd 2 (dba) 3 , Xantphos, the mass ratio of potassium carbonate are 10:4~6:1~3:1~2:8~10, and the solid-liquid g / mL ratio of compound A and dioxane is 1:16, and the material is taken, Add compound A, tert-butyl carbamate, Pd to the reaction flask 2 (dba) 3 , Xantphos, potassium carbonate and dioxane, replaced by nitrogen 3 times, protected by nitrogen, heated to 85°C, and reacted for 12 hours to obtain compound B;
[0021] (2) According to compound B, triethylamine, Pd(dppf)Cl 2 The mass ratio of compound B and methanol is 5:5~7:0.3~1, the solid-liquid g / mL ratio of compound B and methanol is 1:20, and the material is taken, and compound B, triethylamine, Pd(dppf)Cl 2 Add methanol and methanol into the autoclave, feed CO into the reaction system, adjust the pressure to 0.7-0.9MPa, heat to 85-95...
Embodiment 1
[0024] A kind of synthetic method of 5-amino-3-cyanopicolinate methyl ester hydrochloride, the method comprises the steps:
[0025] (1) Add 10g of compound A, 6g of tert-butyl carbamate, 2g of Pd into the reaction flask 2 (dba) 3 , 1.5g Xantphos, 9g potassium carbonate and 160mL dioxane, nitrogen replacement 3 times, nitrogen protection, heated to 85°C, reacted for 12h, TLC detection, the reaction of the raw materials was completed, the reaction system was cooled to room temperature, filtered with a small amount of diatomaceous earth, Rinse the filter cake (80 mL) with ethyl acetate, spin the filtrate to dry, add ethyl acetate (200 mL), wash with saturated brine (80 mL), concentrate, mix the sample and pass through the column to obtain 10.8 g of yellow solid, compound B, yield 92.6% , with a purity of 96.2%;
[0026] (2) Mix 5g compound B, 6g triethylamine, 0.5g Pd(dppf)Cl 2 Add 100mL of methanol into the autoclave, feed CO into the reaction system, adjust the pressure to 0...
Embodiment 2
[0030] A kind of synthetic method of 5-amino-3-cyanopicolinate methyl ester hydrochloride, the method comprises the steps:
[0031] (1) Add 10g compound A, 5g tert-butyl carbamate, 1gPd to the reaction flask 2 (dba) 3 , 2gXantphos, 10g potassium carbonate and 160mL dioxane, nitrogen replacement 3 times, nitrogen protection, heated to 85°C, reacted for 12h, TLC detection, the reaction of the raw materials was completed, the reaction system was cooled to room temperature, filtered with a small amount of diatomaceous earth, acetic acid The filter cake (80 mL) was rinsed with ethyl ester, the filtrate was spin-dried, ethyl acetate (200 mL) was added, washed with saturated brine (80 mL), concentrated, mixed and passed through the column to obtain 10.3 g of a yellow solid. Compound B was obtained with a yield of 88.3% and a purity of 96.8%;
[0032] (2) Combine 5g of compound B, 7g of triethylamine, 1g of Pd(dppf)Cl 2 Add 100mL of methanol into the autoclave, feed CO into the rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com