9-hydroxyphenanthrenequinone derivative as well as preparation method and application thereof
A technology for hydroxyphenanthrenequinone and derivatives, which is applied in the field of 9-hydroxyphenanthrenequinone derivatives and their preparation, can solve the problems that antitumor drugs cannot meet the treatment requirements and lack effective tumor treatment, and achieves high atom economy and good antitumor performance. Effect, novel structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 Preparation of 9-hydroxyphenanthrenequinone derivatives
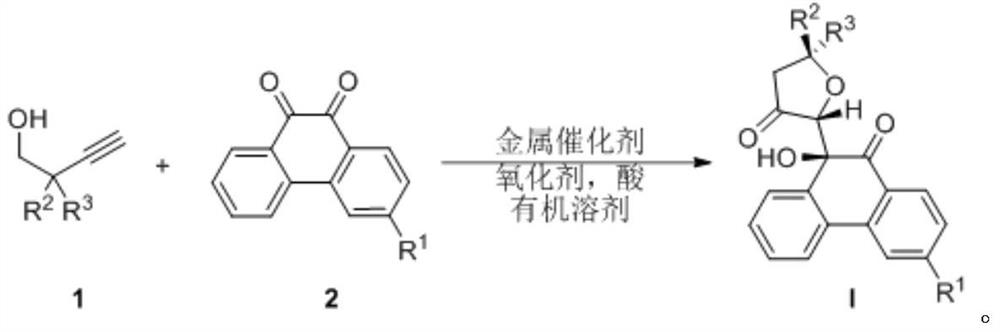
[0045] The preparation method of 9-hydroxyphenanthrenequinone derivative is carried out according to the following reaction formula:
[0046]
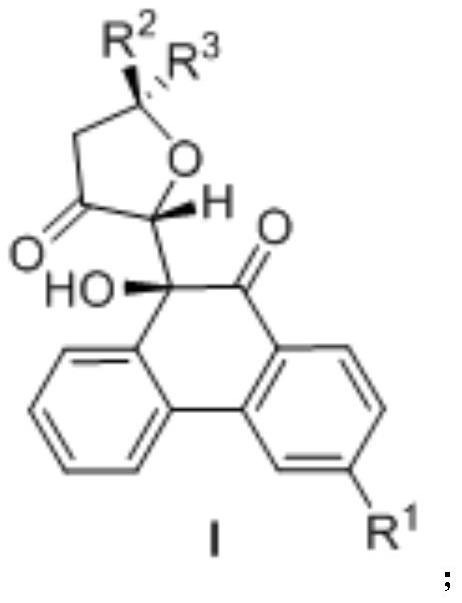
[0047] In the formula, R 1 For hydrogen, bromine, etc.; R 2 For hydrogen, methyl, etc.; R 3 It is hydrogen, benzene, thiophene, furan, isobutyl, phenethyl, halogenated phenyl, methyl substituted phenyl, tert-butyl substituted phenyl, etc.
[0048] Homoacetylenic alcohol (0.60mmol) shown in formula 1 in the above reaction formula, phenanthrenequinone (0.4mmol) shown in formula 2, [2-(dicyclohexylphosphine)-3,6-dimethoxy-2' ,4',6'-triisopropyl-1,1'-diphenyl] bis(trifluoromethanesulfonimide) gold catalyst (0.02mmol), oxidizing agent (its structural formula is shown in O1, 0.60mmol) and trifluoromethanesulfonic acid (0.80mmol) were weighed in a test tube, then 10mL of anhydrous 1,2-dichloroethane was added to the reaction system, and the reaction was stirred at...
Embodiment 2
[0068] Example 2 Inhibitory activity of 9-hydroxyphenanthrenequinone derivatives on small cell lung cancer cells
[0069] 1. Human small cell lung cancer cells and tumor cells used in the determination are: human small cell lung cancer cells (H446) and human small cell lung cancer cells (H128).
[0070] 2. Using the CCK-8 method to determine the inhibitory effect of 9-hydroxyphenanthrenequinone derivatives on the proliferation of human small cell lung cancer cells, wherein the specific determination process of H446 cells and H128 cells is as follows:
[0071] (1) Make single-cell suspensions of H446 and H128 human small cell lung cancer cell lines respectively, take 100 μL and inoculate them in 96-well culture plates, the concentration of single-cell suspensions is 3000 cells / well, and then place in CO 2 in an incubator (37°C, 5% CO 2 , 95% air) overnight.
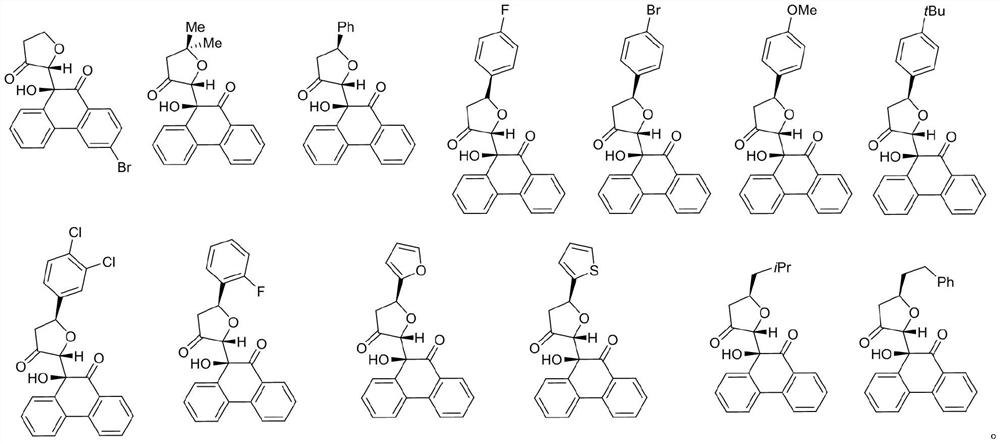
[0072] (2) Dissolve 9-hydroxyphenanthrenequinone derivatives (compounds I-1 to I-13) in DMSO respectively, prepare 10 ...
Embodiment 3
[0083] Example 3 Inhibitory activity of 9-hydroxyphenanthrenequinone derivatives on non-small cell lung cancer cells
[0084] 1. The human non-small cell lung cancer cell tumor cell used in the determination is: human non-small cell lung cancer cell (A549).
[0085] 2. The CCK-8 method was used to measure the inhibitory effect of 9-hydroxyphenanthrenequinone derivatives on the proliferation of human non-small cell lung cancer cells (A549). The specific measurement process was as follows:
[0086] (1) Make a single cell suspension of A549 human small cell lung cancer cell line, take 100 μL and inoculate it in a 96-well culture plate, the concentration of the single cell suspension is 6000 cells / well, and then place in CO 2 in an incubator (37°C, 5% CO 2 , 95% air) overnight culture;
[0087] (2) Dissolve 9-hydroxyphenanthrenequinone derivatives (compounds I-1 to I-13) in DMSO to prepare a 3.3mM stock solution, then dilute it with a blank medium to a concentration of 10μM, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com