Menadione structure-based novel coronavirus 3CL protease inhibitor
A technology of menadione and naphthoquinone, which is applied in the field of medicine, can solve the problems of limited clinical application and strong toxicity, and achieve the effects of easy-to-obtain raw materials, low toxicity, and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
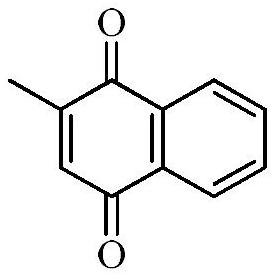
[0029] This embodiment relates to a preparation method of 2-methyl-1,4-naphthoquinone (II-1) having structural formula (II), comprising the following steps:
[0030]
[0031] At room temperature, 14.2g of 2-methylnaphthalene (100mmol) was dissolved in glacial acetic acid (50mL), and this solution was added dropwise to 200 ml of glacial acetic acid solution of 58.7 grams of chromic anhydride. Maintain at 35-40°C. After the addition, keep it at 40°C for 0.5 hours, raise the temperature to 65°C and keep it for 20 minutes; pour the reactant into a large amount of ice water, and precipitate the crude 2-methyl-1,4-naphthoquinone under constant stirring. Filter the crude product, and wash the filter cake repeatedly with ice water until the filtrate has no sour taste. The filter cake was dissolved with dichloromethane, and after the organic layer was separated, a small amount of activated carbon was added for decolorization. The dichloromethane was distilled off under reduced pre...
Embodiment 2
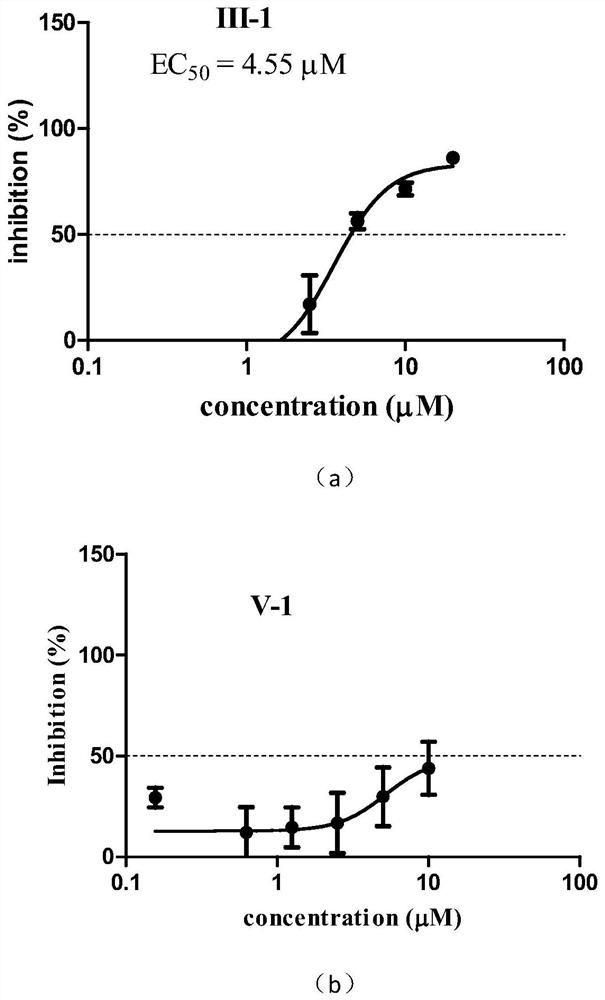
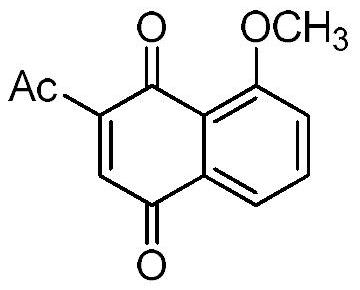
[0033]This example relates to a preparation method of 2-acetyl-8-methoxy-1,4-naphthoquinone (III-1) having the structural formula (III), comprising the following steps:
[0034]
[0035] Using the reported method (Zhang et al.Synthesis of 4,8-dimethoxy-1-naphtholvia an acetyl migration.Synth.Commun.,2017,47(6),536-540), using juglone as raw material, synthesize 4 ,8-Dimethoxy-1-naphthol acetate. Dissolve 4,8-dimethoxy-1-naphthol acetate (300mg, 1.22mmol) in boron trifluoride-ether solution (5mL, boron trifluoride content is 48%), and the reaction solution The temperature was raised to 60°C and the reaction was stirred at this temperature for 30 minutes. The reaction solution was cooled and diluted with ice water, extracted with dichloromethane, the organic layer was dried and concentrated to dryness under reduced pressure, and the residue was purified by column chromatography to obtain 2-acetyl-4,8-dimethoxy-1- Naphthol, light yellow powder, about 237mg, yield: 79%. 1 H ...
Embodiment 3
[0037] This embodiment relates to a preparation method of 2-hydroxy-1,4-naphthoquinone (IV-1) having structural formula (IV), comprising the following steps:
[0038]
[0039] 1,4-Naphthoquinone (3.16 g, 20 mmol) was dissolved in acetic anhydride (20 mL), and 4 drops of concentrated sulfuric acid were added dropwise. The mixture was stirred and reacted in an ice-water bath for 8 hours. The reaction solution was filtered with suction, and the filter cake was washed with petroleum ether and a small amount of pre-cooled absolute ethanol to obtain about 4.72 g of off-white powder. The powder was dissolved in methanol, and 0.5 g of sodium methoxide was added under an ice-water bath. After the mixture was stirred and reacted in an ice-water bath for 4 hours, it was suction-filtered, and the red filter cake was collected and washed with a small amount of methanol. Dissolve the above filter cake in water at 90°C, and suction filter it while it is hot; acidify the filtrate with con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com