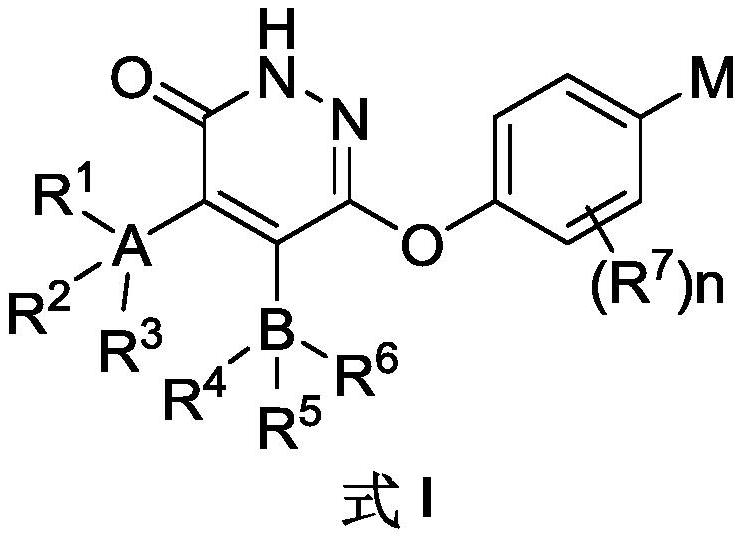
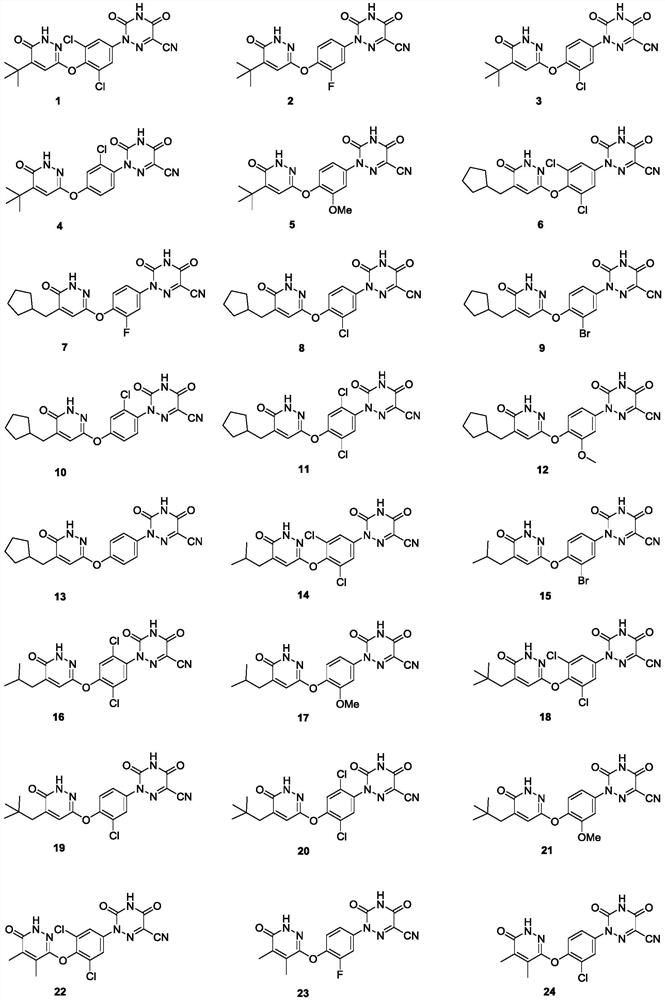
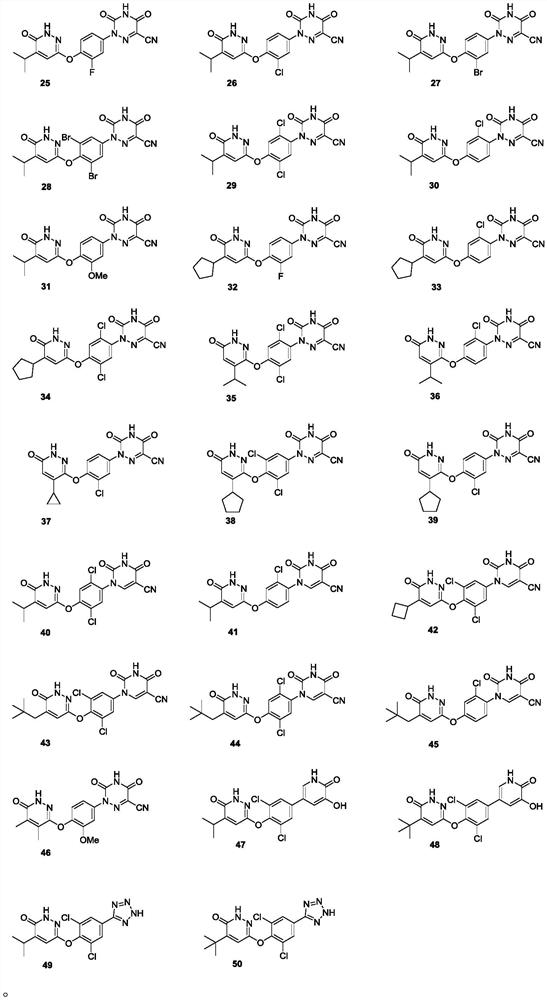
Substituted pyridazinone compound and application thereof
A compound and representative technology applied in the field of medicinal chemistry to achieve strong THβ selectivity and good pharmacokinetic parameters
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0045] Synthesis of 2-(3,5-Dichloro-4-(5-isopropyl-6-oxo-1,6-dihydropyridazin-3-oxy)phenyl)-3,5-dioxo -2,3,4,5-Tetrahydro-1,2,4-triazine-6-carbonitrile (1)
[0046]
[0047] 1. Synthesis of 3,6-dichloro-4-tert-butylpyridazine (1-1)
[0048] In a 1L round-bottom flask were added 3,6-dichloropyridazine (14.9 g, 0.1 mol), pivalic acid (20.4 g, 0.2 mol), purified water (300 mL) and trifluoroacetic acid (7.4 mL, 0.1 mol) , the temperature was raised to 70 °C, silver nitrate (3.4 g, 0.2 mol) was added, and finally an aqueous solution of ammonium persulfate (91.3 g dissolved in 200 mL of water) was added dropwise. The reaction was carried out at 70°C for 30 min. TLC showed that the reaction of the raw materials was complete. The reaction was stopped, and the reaction solution was lowered to room temperature. The pH was adjusted to 9-10 with concentrated ammonia water, extracted with ethyl acetate (200 mL×3), and the organic phases were combined, and the organic phases were satura...
experiment example 2
[0059] Synthesis of 2-(4-((5-(tert-butyl)-6-oxo-1,6-dihydropyridazin-3-yl)oxy)-3-fluorophenyl)-3,5-di Oxy-2,3,4,5-tetrahydro-1,2,4-triazine-6-carbonitrile (2)
[0060]
[0061] 1. Synthesis of N-(3-fluoro-4-hydroxyphenyl)acetamide (2-1)
[0062] A 100 mL round-bottom flask was charged with 4-amino-2-fluorophenol (5.08 g, 40 mmol), acetic anhydride (4.08 g, 40 mmol) and glacial acetic acid (30 mL), and stirred at room temperature overnight. The solid obtained after the solvent was spin-dried was rinsed with ethyl acetate for several times, placed in an oven and dried at 50 °C for 4 h to obtain the target product 2-1 as a brown solid (6.1 g, 90%). LRMS:C 8 H 9 FNO 2 (M+H) + m / z = 170.1, molecular weight = 169.1554, exact mass = 169.0539.
[0063] 2. Synthesis of N-(4-((5-(tert-butyl)-6-chloropyridin-3-yl)oxy)-3-fluorophenyl)acetamide (2-2)
[0064] In a 100 mL round-bottomed flask, compound 2-1 (0.62 g, 3.0 mmol), 2,6-dichloro-4-aminophenol (0.51 g, 3.0 mmol) and potas...
experiment example 3
[0073] Synthesis of 1-(3,5-Dichloro-4-((5-cyclobutyl-6-oxo-1,6-dihydropyridazin-3-yl)oxy)phenyl)-2,4- Dioxy-1,2,3,4-tetrahydropyrimidine-5-carbonitrile (42)
[0074]
[0075] 1. Synthesis of (2-cyano-3-methoxyacryloyl) urethane (42-1)
[0076] Ethyl carbamate (3.12 g, 20 mmol) and 40 mL of acetonitrile were added to a 100 mL eggplant flask, and after stirring to dissolve, trimethyl orthoformate (3.5 mL, 40 mmol) and acetic anhydride (20 mL) were added. The reaction solution was heated to 80°C and reacted for 4 hours, the reaction was stopped, and 50 mL of ether was added to dry the solvent to obtain a suspension, which was kept in a refrigerator at 2-8°C overnight. The suspension was filtered, and the filter cake was rinsed with ether for several times, placed in an oven and dried at 50°C for 4 h to obtain the target product 42-1 as a white solid (2.2 g, 56%). LRMS:C 8 H 11 N 2 O 4 (M+H) + m / z=199.1, molecular weight=198.1780, exact mass=198.0641.
[0077] 2. Synth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com