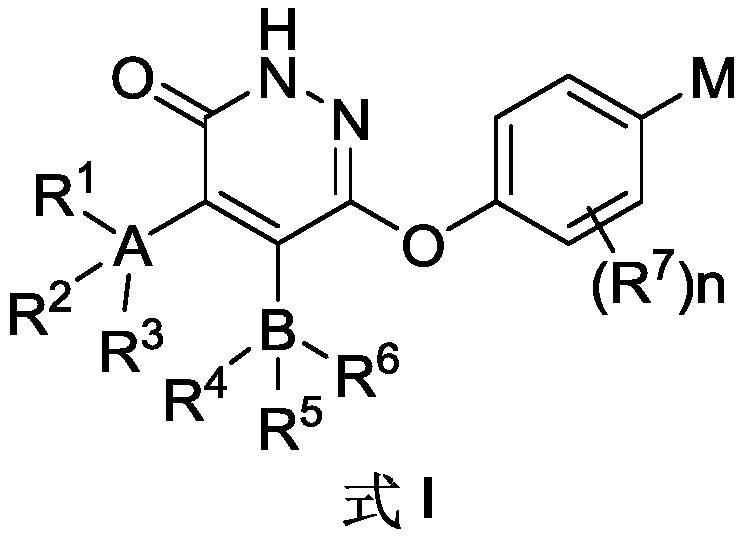
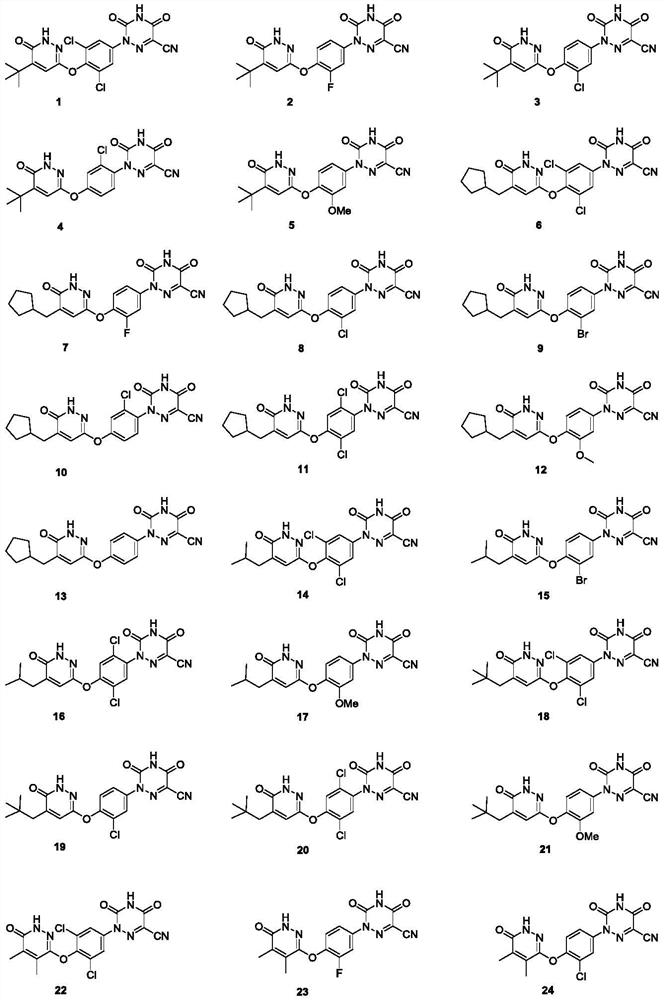
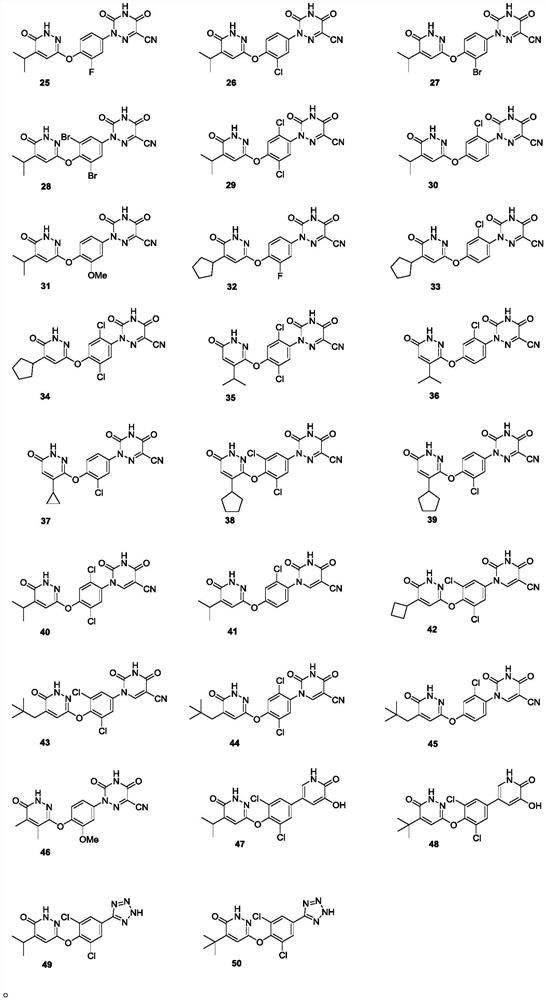
Substituted pyridazinones and uses thereof
The technology of a compound, pyridazinone, is applied in the field of medicinal chemistry to achieve the effects of increased selectivity, enhanced agonistic activity, and good pharmacokinetic parameters
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0045] Synthesis of 2-(3,5-dichloro-4-(5-isopropyl-6-oxo-1,6-dihydropyridazin-3-oxyl)phenyl)-3,5-dioxo -2,3,4,5-Tetrahydro-1,2,4-triazine-6-carbonitrile (1)
[0046]
[0047] 1. Synthesis of 3,6-dichloro-4-tert-butylpyridazine (1-1)
[0048] Add 3,6-dichloropyridazine (14.9 g, 0.1 mol), pivalic acid (20.4 g, 0.2 mol), purified water (300 mL) and trifluoroacetic acid (7.4 mL, 0.1 mol) into a 1 L round bottom flask , the temperature was raised to 70° C., silver nitrate (3.4 g, 0.2 mol) was added, and finally ammonium persulfate aqueous solution (91.3 g dissolved in 200 mL of water) was added dropwise. Reacted at 70°C for 30 minutes, TLC showed that the raw materials were completely reacted, stopped the reaction, cooled the reaction solution to room temperature, adjusted the pH to 9-10 with concentrated ammonia water, extracted with ethyl acetate (200mL×3), combined the organic phases, and used saturated Wash with sodium chloride and dry with anhydrous sodium sulfate. Conce...
experiment example 2
[0059] Synthesis of 2-(4-((5-(tert-butyl)-6-oxo-1,6-dihydropyridazin-3-yl)oxy)-3-fluorophenyl)-3,5-di Oxy-2,3,4,5-tetrahydro-1,2,4-triazine-6-carbonitrile (2)
[0060]
[0061] 1. Synthesis of N-(3-fluoro-4-hydroxyphenyl)acetamide (2-1)
[0062] Add 4-amino-2-fluorophenol (5.08 g, 40 mmol), acetic anhydride (4.08 g, 40 mmol) and glacial acetic acid (30 mL) into a 100 mL round bottom flask, and stir overnight at room temperature. The solid obtained after the solvent was spin-dried was washed with ethyl acetate several times, and placed in an oven at 50°C for 4 h to obtain the target product 2-1 as a brown solid (6.1 g, 90%). LRMS:C 8 h 9 FNO 2 (M+H) + m / z = 170.1, molecular weight = 169.1554, exact mass = 169.0539.
[0063] 2. Synthesis of N-(4-((5-(tert-butyl)-6-chloropyridin-3-yl)oxy)-3-fluorophenyl)acetamide (2-2)
[0064] Add compound 2-1 (0.62g, 3.0mmol), 2,6-dichloro-4-aminophenol (0.51g, 3.0mmol) and potassium carbonate (0.83g, 6.0mmol) in 100mL round bottom fl...
experiment example 3
[0073] Synthesis of 1-(3,5-dichloro-4-((5-cyclobutyl-6-oxo-1,6-dihydropyridazin-3-yl)oxy)phenyl)-2,4- Dioxy-1,2,3,4-tetrahydropyrimidine-5-carbonitrile (42)
[0074]
[0075] 1. Synthesis of (2-cyano-3-methoxyacryloyl) ethyl carbamate (42-1)
[0076] Add urethane (3.12g, 20mmol) and 40mL acetonitrile in 100mL eggplant-shaped flask, after stirring to dissolve, add trimethyl orthoformate (3.5mL, 40mmol) and acetic anhydride (20mL). The reaction solution was heated to 80°C for 4 hours, and the reaction was stopped. After the solvent was spin-dried, 50 mL of diethyl ether was added to obtain a suspension, which was left to stand overnight in a refrigerator at 2-8°C. The suspension was filtered, the filter cake was rinsed with diethyl ether several times, and placed in an oven at 50°C for 4 h to obtain the target product 42-1 as a white solid (2.2 g, 56%). LRMS:C 8 h 11 N 2 o 4 (M+H) + m / z = 199.1, molecular weight = 198.1780, exact mass = 198.0641.
[0077] 2. Synthesi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com