Betamethasone fluorination production process
A betamethasone and production process technology, applied in the direction of steroids, organic chemistry, etc., can solve the problems of low content and yield of betamethasone, large amount of hydrogen fluoride, small reaction volume, etc., and achieve shortening of neutralization time and yield The effect of improving efficiency and quality and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
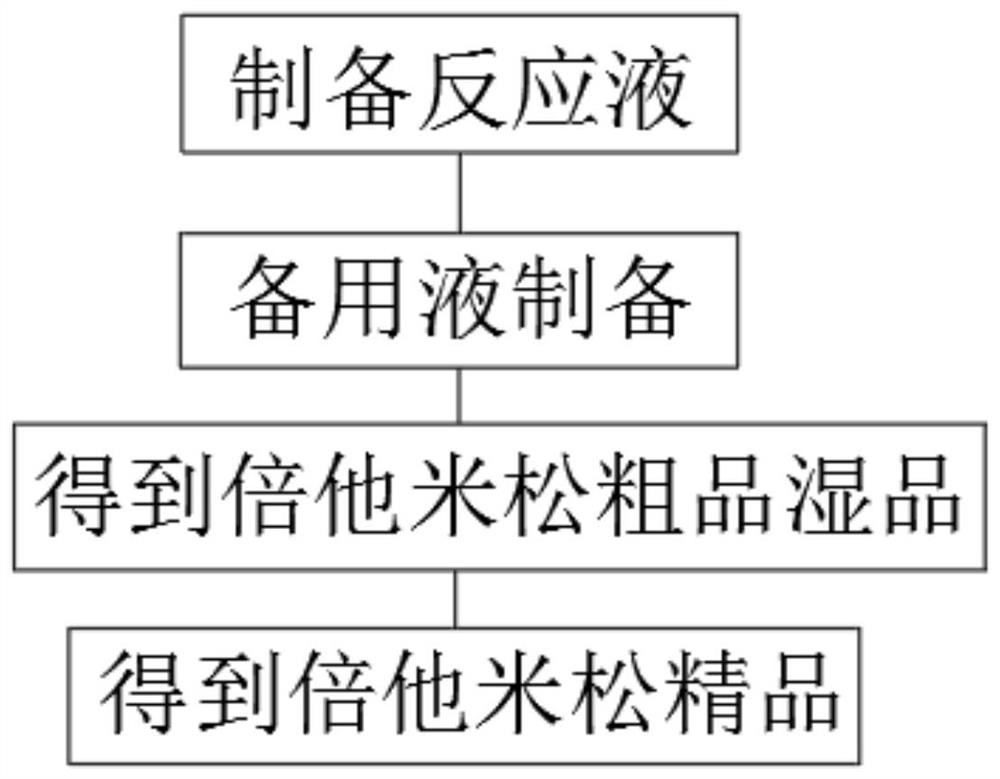
[0027] see figure 1 , a process for producing fluorine on betamethasone, comprising the following steps:
[0028] S1: Under the protection of nitrogen, add 30g of betamethasone epoxy and 150ml of acetone into the reaction flask, stir and cool down, keep the temperature at -5~0℃, control the temperature <5℃, add hydrofluoric acid-fluorination Potassium solution (0.18g of potassium fluoride dissolved in 3.3g of hydrofluoric acid), after the dropwise addition, was kept at 0-10°C for 2.5h to obtain a reaction solution;
[0029] S2: Preparation of reserve solution: Add 30ml of drinking water to a beaker, slowly add 12g of potassium hydroxide, stir evenly, exotherm violently during the preparation process, cool to 0-5°C for use, and obtain a reserve solution.
[0030] S3: TLC detects that the reaction of the raw material betamethasone epoxy is complete, slowly dilute it in 450ml of water, then add the reserve solution dropwise, control the temperature not to exceed 20°C, add dropwi...
Embodiment 2
[0033] see figure 1 , a process for producing fluorine on betamethasone, comprising the following steps:
[0034] S1: Under the protection of nitrogen, add 30g of betamethasone epoxy and 150ml of acetone into the reaction flask, stir and cool down to make the temperature -5~0°C, control the temperature <5°C, add dropwise hydrofluoric acid solution (0.15 g of potassium fluoride dissolved in 3 g of hydrofluoric acid), after the dropwise addition is completed, the temperature is kept at 0-10° C. for 3 hours to obtain a reaction solution;
[0035] S2: Preparation of reserve solution: add 30ml of drinking water to a beaker, slowly add 12g of potassium hydroxide, stir evenly, exotherm violently during the preparation process, cool to 0-5°C for use, and obtain a reserve solution;
[0036] S3: TLC detects that the reaction of the raw material betamethasone epoxy is complete, slowly dilute it in 450ml of water, then add the reserve solution dropwise, control the temperature not to exc...
Embodiment 3
[0039] see figure 1 , a process for producing fluorine on betamethasone, comprising the following steps:
[0040] S1: Under the protection of nitrogen, add 30g of betamethasone epoxy and 120ml of recovered acetone into the reaction flask, stir and cool down to make the temperature -5 ~ 0°C; control the temperature <5°C, add dropwise hydrofluoric acid solution ( Dissolve 0.3g of potassium fluoride in 4.5g of hydrofluoric acid), after the dropwise addition, keep warm at 0-10°C for 1.5h to obtain a reaction solution.
[0041] S2: Preparation of reserve solution: add 30ml of drinking water to a beaker, slowly add 12g of potassium hydroxide, stir evenly, exotherm violently during the preparation process, cool to 0-5°C for use, and obtain a reserve solution;
[0042] S3: TLC detects that the raw material betamethasone epoxy reacts completely, slowly dilute it in 360ml of water, then add the reserve solution dropwise, control the temperature not to exceed 20°C, add dropwise until th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com