NMN slow-release enteric microcapsule and preparation method thereof
A microcapsule and enteric-coated technology, which is applied in the field of NMN slow-release enteric-coated microcapsules and its preparation, achieves high yield, good product stability, and improved bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] The present invention also provides a preparation method of NMN slow-release enteric-coated microcapsules, the method comprising:
[0054] S1. Disperse NMN, emulsifier and other oil-soluble components in the auxiliary agent in the carrier oil to form an oil phase mixture, and the oil phase mixture is atomized and adsorbed on the adsorbent to form core particles; the wall material I and The water-soluble components in the wall material II and other additives are dissolved in water to form an aqueous phase solution, which is heated to 40-50°C and kept warm for later use; the wall material I is non-protein carbohydrate and / or vegetable glue wall material, so The wall material II is a wall material having an electric charge opposite to that of the emulsifier, and the other additives are selected from at least one of antioxidants, colorants, sunscreens and food flavors and fragrances;
[0055] S2, mixing the core particles with the aqueous phase solution, shear emulsificatio...
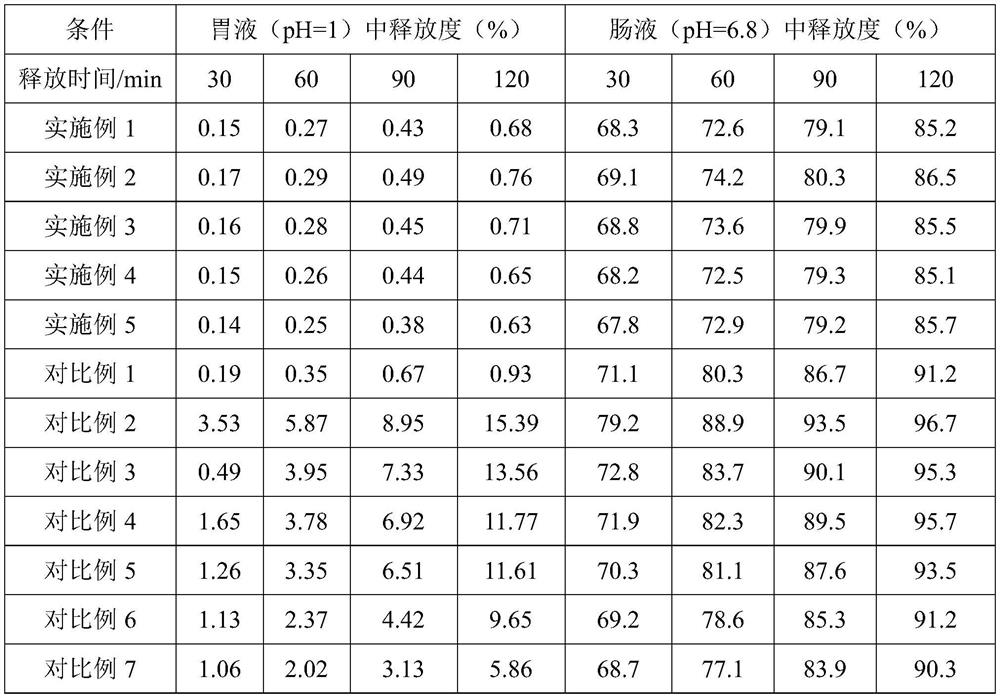
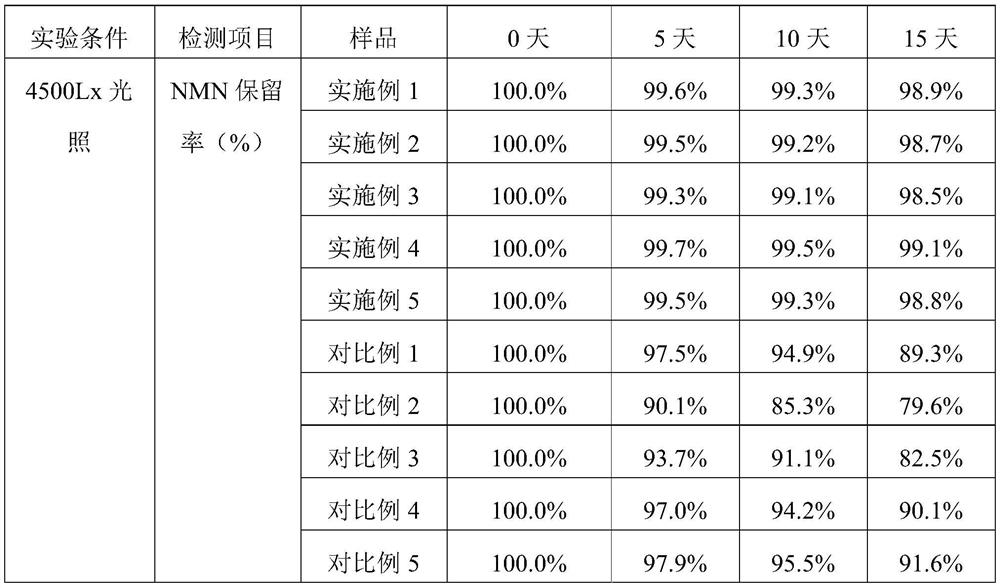
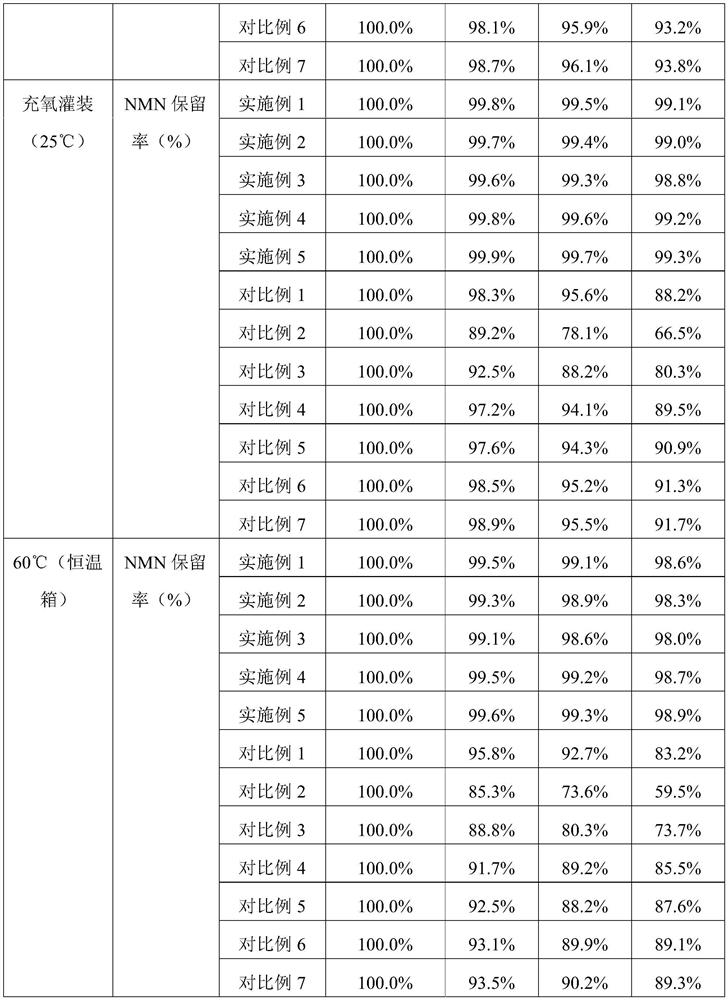
Embodiment 1
[0065] Formula composition
[0066] recipe ingredients Feed amount (weight part) NMN 1 MCT 5 Calcium stearoyl lactylate 0.1 fumed silica 10 Modified starch (Cargill 12670) 40 Maltodextrin 39.9 Chitosan 4
[0067] S1. Uniformly disperse 1 part of NMN pure product and 0.1 part of calcium stearoyl lactylate in 5 parts of carrier oil MCT through a nano grinder to form an oil phase mixture, and atomize the oil phase mixture on 10 parts of fumed silica adsorbent Adsorbed on top to form core particles; dissolve 40 parts of modified starch, 39.9 parts of maltodextrin, and 4 parts of chitosan in 110 parts of pure water at 40-50°C, mix well to prepare an aqueous phase solution, and store at 40-50°C The lower heat preservation spare;
[0068] S2. Mix the core particles and the aqueous solution, and shear for 15 minutes at a speed of 10,000 rpm to obtain a stable NMN emulsion;
[0069] S3. Perform low-temperature electrostatic spr...
Embodiment 2
[0071] Formula composition
[0072] recipe ingredients Feed amount (weight part) NMN 1 MCT 20 Calcium stearoyl lactylate 0.5 fumed silica 20 Modified starch (Cargill 12670) 40 Maltodextrin 14.5 Chitosan 4
[0073] S1. Uniformly disperse 1 part of NMN pure product and 0.5 part of calcium stearoyl lactylate in 20 parts of carrier oil MCT through a nano grinder to form an oil phase mixture, and atomize the oil phase mixture on 20 parts of fumed silica adsorbent Adsorbed on top to form core particles; dissolve 40 parts of modified starch, 14.5 parts of maltodextrin, and 4 parts of chitosan in 110 parts of pure water at 40-50 °C, mix well to prepare an aqueous phase solution, and store at 40-50 °C The lower heat preservation spare;
[0074] S2. Mix the core particles and the aqueous solution, and shear for 15 minutes at a speed of 10,000 rpm to obtain a stable NMN emulsion;
[0075] S3. Perform low-temperature electrostatic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com