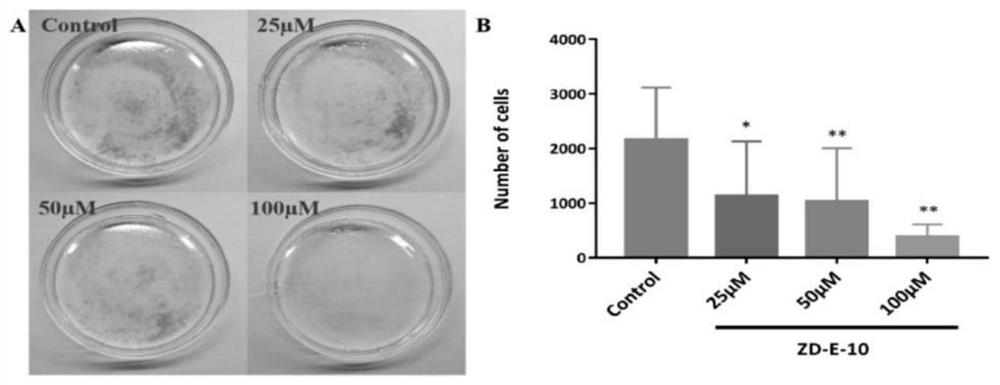
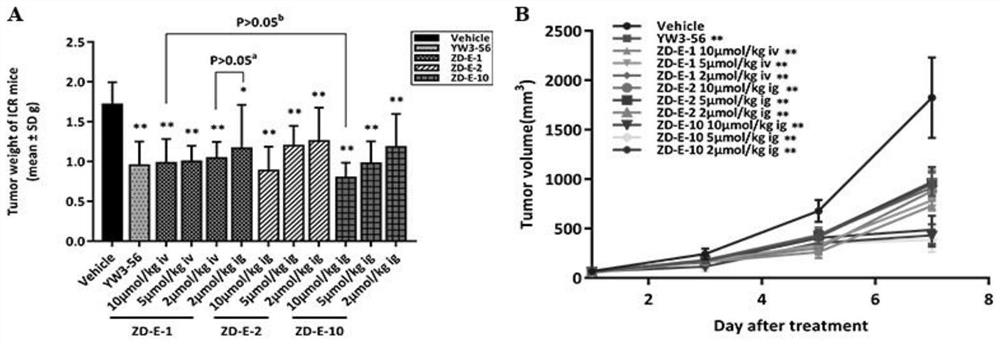
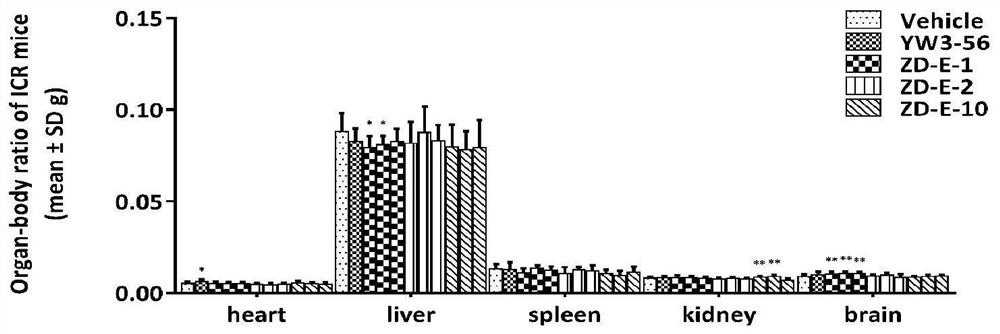
Small molecule pad4 inhibitor and its preparation method and application
A technology of small molecules and inhibitors, applied in the field of small molecule PAD4 inhibitors and its preparation, can solve the problems of lack of oral activity, poor bioavailability, unsatisfactory activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0079] In the present invention, when R 1 for R 2 for When, the preparation method of described small molecule PAD4 inhibitor comprises the following steps:
[0080] Dissolve the first reaction raw material in tetrahydrofuran, mix the resulting mixture with 1-hydroxybenzotriazole and dicyclohexylcarbodiimide, and perform the first activation, then mix the resulting activation system with benzylamine, and use N- Methylmorpholine adjusts the pH value of the obtained mixed solution to 8-9, performs the first condensation reaction, and obtains the first intermediate product;
[0081] Dissolving the first intermediate product in ethyl acetate, mixing the resulting mixture with HCl in ethyl acetate, and then performing a first hydrolysis reaction to obtain a second intermediate product;
[0082] Dissolving the second reaction raw material in tetrahydrofuran, mixing the resulting mixture with 1-hydroxybenzotriazole and dicyclohexylcarbodiimide, and then performing the second ...
Embodiment 1
[0131] The synthesis of embodiment 1 (3-boronobenzoic acid)-Orn (Cl)-NBzl (ZD-E-2)
[0132] (1) Synthesis of Boc-Orn(Z)-NBzl
[0133] Weight loss method Accurately weigh 3.660g (10mmol) Boc-Orn(Z)-OH and place it in an eggplant bottle, dissolve it with 100mL anhydrous THF until it is colorless, clear and transparent, add a stirrer; under ice bath stirring, add 1.620g (12mmol) 1-hydroxybenzotriazole (HOBt) and 2.472g (12mmol) dicyclohexylcarbodiimide (DCC), after activating 10min, white solid is separated out; Add 1.6mL benzylamine dropwise in eggplant bottle, And use N-methylmorpholine (NMM) to adjust the pH to 8; remove the ice bath, stir and react at room temperature (25°C) for 8h, and use TLC (by volume, the developing agent is CH 2 Cl 2 :CH 3 OH=30:1,R f =0.35) detect the reaction process, observe the disappearance of the raw material point, and judge that the reaction is complete. After the reaction, use a vacuum circulating water pump to filter under reduced pressur...
Embodiment 2
[0142] The synthesis of embodiment 2 (2-boronobenzoic acid)-Orn (Cl)-NBzl (ZD-E-3)
[0143] (1) Synthesis of (2-boronobenzoic acid)-Orn(Z)-NBzl
[0144] The target product was prepared with reference to the method of step (3) in Example 1, except that "3-carboxyphenylboronic acid (3-boronobenzoic acid)" was replaced by "2-carboxyphenylboronic acid (2-boronobenzoic acid)", and finally 1.650 g (65.6%) of the target product (2-boronobenzoic acid)-Orn(Z)-NBzl were obtained as a white solid. ESI-MS(m / z):538.6[M+Cl] - ; 1 H-NMR (300MHz, DMSO-d 6 ): δ(ppm)=8.78(t, J=5.7Hz, 1H), 7.76(dt, J=7.7Hz, 1.9Hz, 1H), 7.57(d, J=7.4Hz, 1H), 7.44(m, 1H),7.41(m,1H),7.38(m,1H),7.30(m,10H),6.88(m,1H),5.01(s,2H),4.28(m,2H),3.97(t,J =6.6Hz, 1H), 3.03(t, J=6.7Hz, 2H), 1.75(m, 2H), 1.50(m, 2H).
[0145] (2) Synthesis of (2-boronobenzoic acid)-Orn-NBzl
[0146] The target product was prepared by referring to the method of step (4) in Example 1, specifically, 1.650g (3.3mmol) (2-boronobenzoic acid)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com