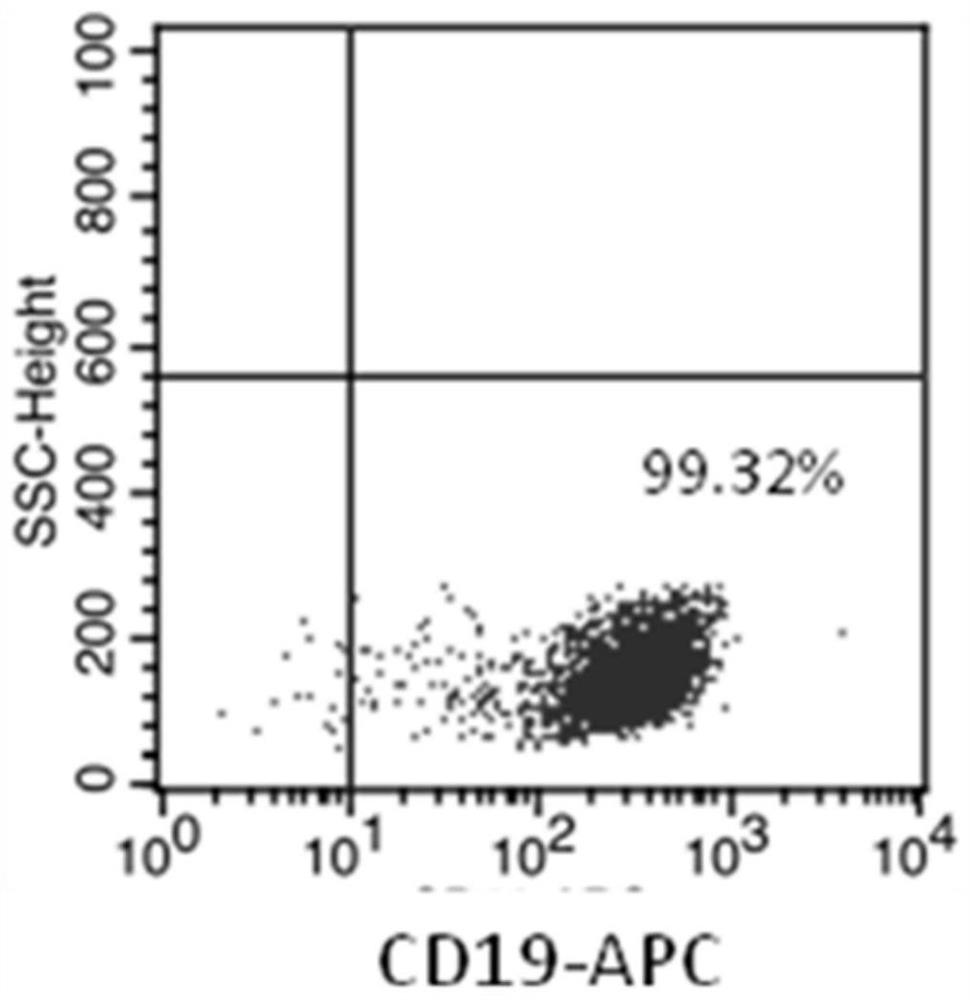
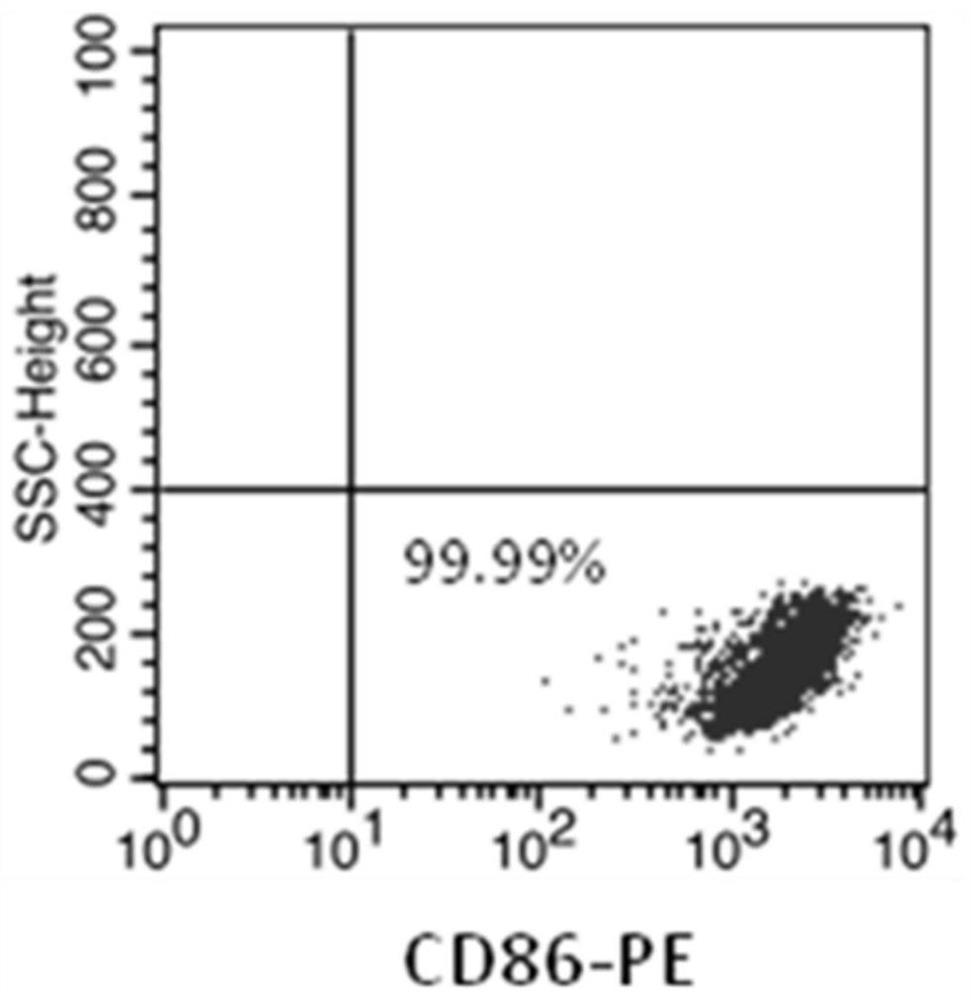
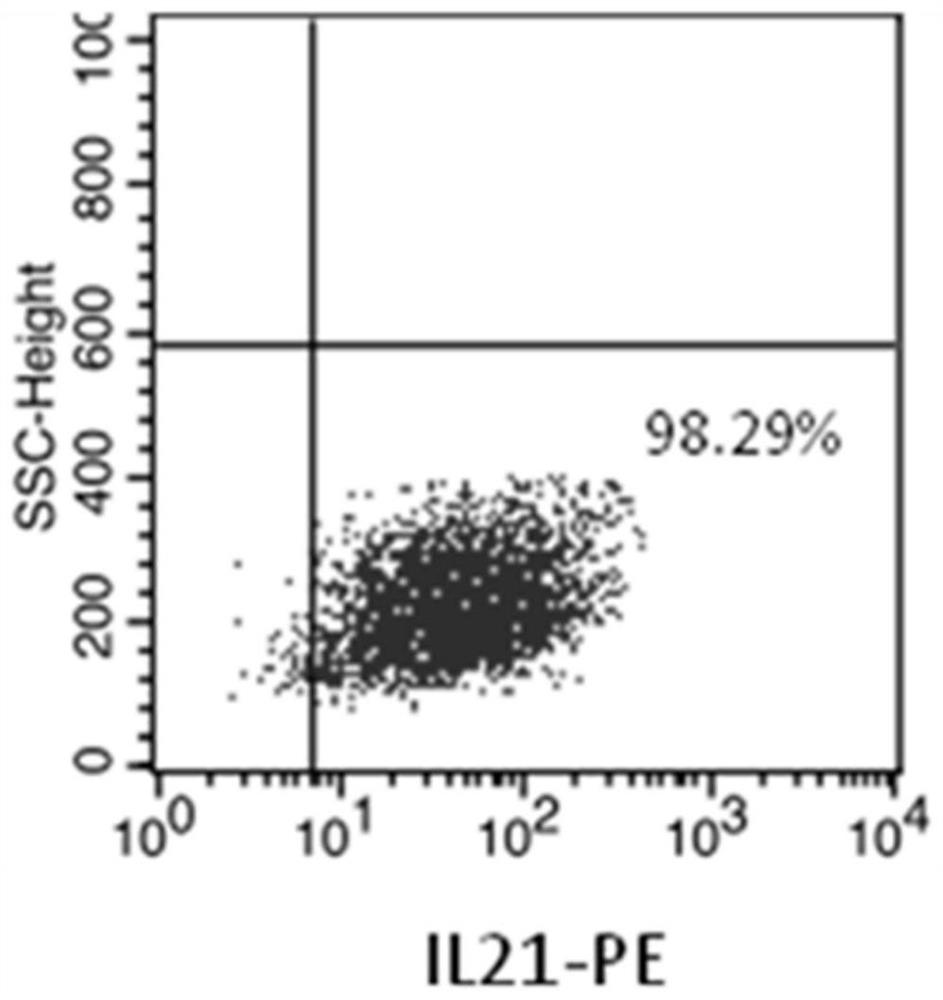
NK cell as well as preparation method and application thereof
A NK cell and cell technology, applied in the field of biomedicine, can solve the problems of high cost, large amount of trophoblast cells, and difficulty in meeting clinical needs in terms of NK amplification quantity, purity and NK cell viability, and achieves low usage and high expansion. The effect of increasing quantity and reducing cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061]In this embodiment, an engineered K562 cell is prepared, and the preparation method comprises:
[0062](1) Artificial Synthesis SEQ ID NO: 1, SEQ ID NO: 2, SEQ ID NO: 3, SEQ ID NO: 4 and SEQ ID NO: 5 Gene Sequence (Saturchero Biological Engineering (Shanghai) Co., Ltd.), and will SEQ ID NO: 1 and SEQ ID NO: 2 Synthesis to a gene sequence, SEQ ID NO: 1 and SEQ ID NO: 2 gene sequence named KCD19CD86, SEQ ID NO: 3 gene sequence named KIL21CD8α, SEQ ID NO: 4 The gene sequence is named KCD64, and the SEQ ID NO: 5 is named CD137L, which connects the synthetic gene sequence to the HLU vector, and the enzyme dug is BamHi / EcoRI or BamHi / NOTI, and the enzyme-cutting reaction system is shown in Table 1, The enzyme digestion was 30 ° C for 1 h, and 1.5 h was 1.5 h at 37 ° C for 1.5 h to give HLU-KCD19CD86, HLU-KCD64 and HLU-CD137L expression vectors;
[0063]Table 1
[0064]
[0065]
[0066](2) A strain containing HLU-KCD19CD86, HLU-KIL21CD8α, HLU-KCD64, HLU-CD137L or LV5-EGFP carrier is performed,...
Embodiment 2
[0072]In this example, a NK cell is prepared, and the method of preparing the NK cell comprises the steps of:
[0073](1) Pulling the anticoagulant cap slit with iodophor, after drying, the anti-condensate cover is opened, and the umbilical cord blood in the anti-concentration pipe is pulled out of the pipette, and the 50 ml of centrifuge tube is added, no more than 35ml per tube. The centrifuge is flattened, 2900r / min is centrifuged for 10 min;
[0074](2) The upper plasma obtained from the center was transferred to 50 ml of centrifuge tube, and the water bath was 30 min, then 1500 r / min was centrifuged to remove precipitation, transfer the upper plasma to a new 50 ml of centrifuge tube, and placed in a 4 ° C refrigerator preservation spare (If there is a precipitation after the refrigerator is placed, then centrifuge again);
[0075](3) Use physiological saline by volume of 2: 1 dilution of cord blood, mix mix;
[0076](4) After the dilution, the blood is carefully added to the separator,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com