A kind of fluoxetine hydrochloride capsule and preparation method thereof
A technology of fluoxetine hydrochloride capsules and fluoxetine hydrochloride, which is applied in the directions of capsule delivery, pharmaceutical formulations, and medical preparations with inactive ingredients, etc., can solve problems such as affecting clinical compliance and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 2
[0061] The preparation of embodiment 1 simethicone emulsion
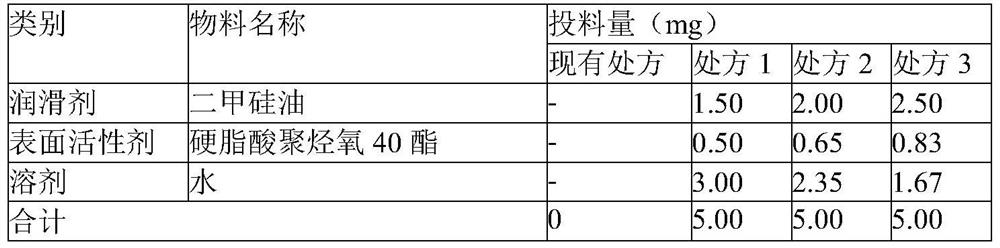
[0062] Pick materials according to the types of materials designed in the recipes shown in Table 2; for the simethicone emulsion, design and weigh the auxiliary materials according to recipes 1 to 3, operate according to the process control parameters in Table 3, and heat the weighed water, and the temperature is not When the temperature exceeds 80 °C, add polyoxy 40 stearate, stir and disperse with a high-shear dispersing emulsifier, and then add dimethicone, and adjust the stirring speed of the high-shear dispersing emulsifier to 5000 rpm to 20,000 rpm to obtain a simethicone emulsion. , it does not delaminate after standing for 24h.
[0063] The prescription screening of table 2 pregelatinized starch lubricant
[0064]
[0065] Table 3 Process Screening of Pregelatinized Starch Lubricants
[0066] Process control parameters Existing prescription prescription 1 prescription 2 prescription 3 ...
Embodiment 2
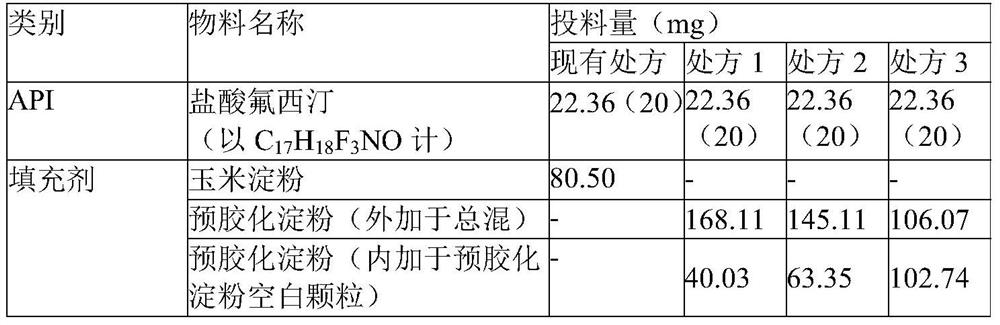
[0067] The preparation of embodiment 2 fluoxetine hydrochloride capsule
[0068] Pick materials according to the types of materials designed in the recipe shown in Table 4, and operate according to the process control parameters in Table 5. The fluoxetine hydrochloride is pulverized, and the particle size distribution D90 is controlled at 20 μm to 100 μm; the pregelatinized starch and silica are passed through a 60-mesh sieve. Weigh the raw materials and excipients according to the design amount of prescription 1-3. Add the pregelatinized starch to the wet granulator, set the stirring speed of the wet granulator to 70 rpm to 100 rpm and the cutting speed to 1200 rpm to 1800 rpm, then start the wet granulator, and use the spray technique to add simethicone emulsion and water for granulation. Then use 6.0mm×6.0mm square-hole screen for wet granulation. After unloading, it is dried in a fluidized bed to measure the moisture content of the material. When the moisture content is l...
Embodiment 3
[0080] Example 3 Comparison of in vitro dissolution curves of fluoxetine hydrochloride capsules (A, B, C) prepared by the present invention, existing product (X) and reference preparation (R)
[0081] Get each 12 capsules of fluoxetine hydrochloride capsules (A, B, C), existing product (X) and reference preparation (R) prepared in Example 2, and detect its hydrochloric acid solution at pH1.0, pH4 according to the following method In vitro dissolution profiles of .5 acetate buffer, pH 6.8 phosphate buffer and water. The detection method is shown in Table 7.
[0082] The method of table 7 fluoxetine hydrochloride capsules in 4 different dissolution media
[0083] Dissolution medium method Rotating speed Surfactant pH1.0 hydrochloric acid solution paddle method 50 rpm none pH4.5 acetate buffer solution paddle method 50 rpm none pH6.8 Phosphate buffer solution paddle method 50 rpm none water paddle method 50 rpm none ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com