Preparation method and application of norcantharidin-loaded exosome
A technology of norcantharidin and exosomes, applied in the field of loaded norcantharidin exosomes and its preparation, can solve the problems of potential toxicity to be verified, adverse reactions, reduced targeting efficiency, etc., and achieve enhanced drug loading , Improve drug efficacy, improve the effect of encapsulation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
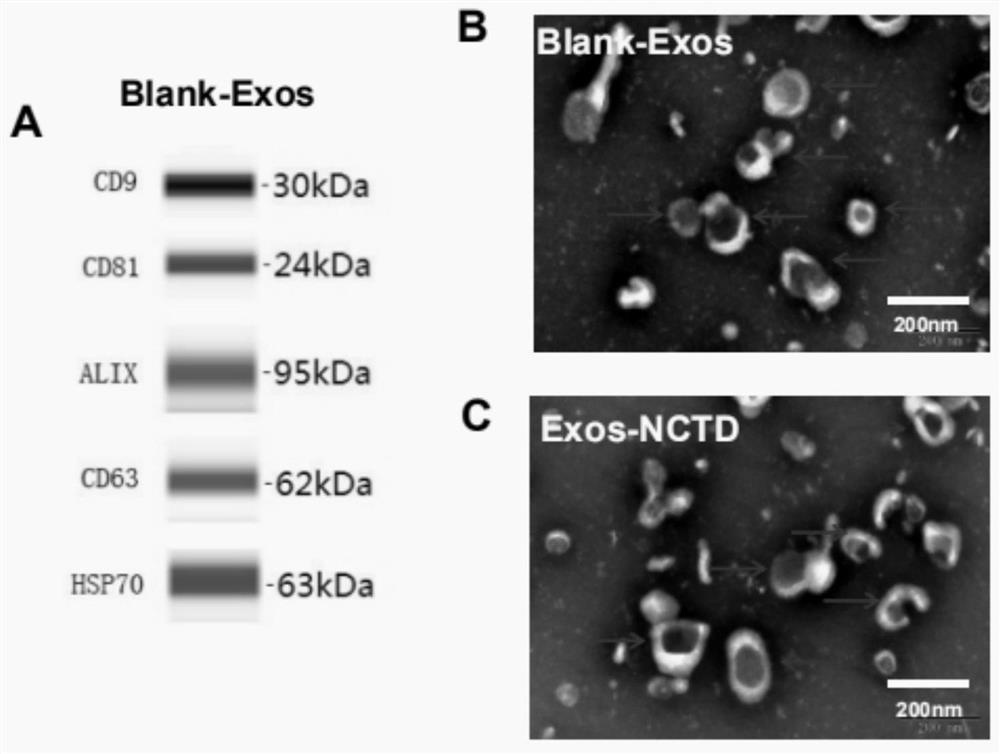
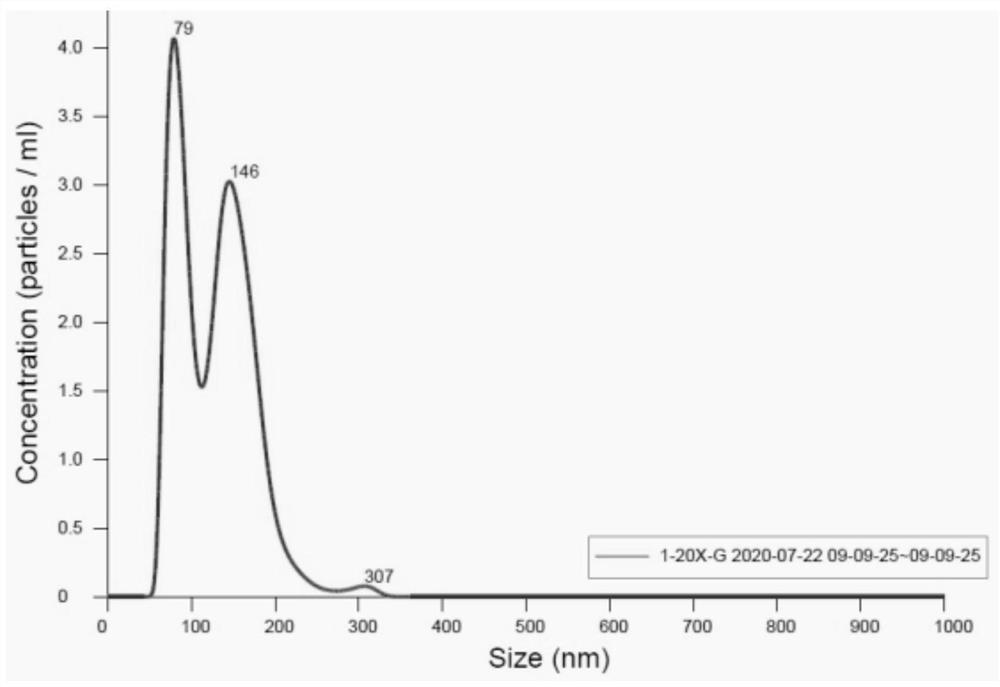
[0067] Example 1: A method for preparing norcantharidin-loaded exosomes and its characterization
[0068] (1) Extraction of exosomes:
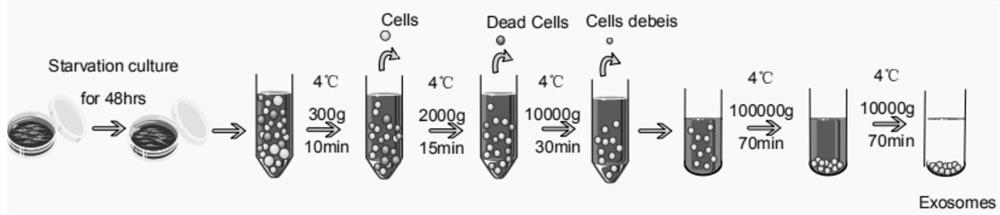
[0069] Exosomes were extracted by differential centrifugation. Bone marrow mesenchymal stem cells (BMSCs) were expanded and cultured in a culture dish. When the cell density reached 80%, the original culture medium containing serum was discarded, washed twice with PBS, then added with DMEM, and starved for 48 hours. Collect DMEM containing exosomes, centrifuge at low temperature for three times to remove macromolecular substances, centrifuge for the first time at 4°C, 300g, centrifuge for 10min, absorb the supernatant, discard the sediment, the purpose is to remove substances such as cells and dead cells; Centrifuge for the second time at 4°C, 2000g for 15min, discard the precipitate, and take the supernatant. The purpose of this centrifugation is to remove large cell debris and other substances; The supernatant, the purpose of this centrifu...
Embodiment 2
[0075] Example 2 A preparation method of loaded norcantharidin exosomes and its property characterization
[0076] (1) Extraction of exosomes:
[0077] Exosomes were extracted by ultracentrifugation. Bone marrow mesenchymal stem cells (BMSCs) were expanded and cultured in a culture dish. When the cell density reached 90%, the original culture medium containing serum was discarded, washed with PBS three times, added DMEM, and starved for 45 hours. Collect the DMEM containing exosomes, centrifuge three times at low temperature to remove macromolecular substances, centrifuge for the first time at 6°C, 400g, centrifuge for 5min, absorb the supernatant, discard the sediment, the purpose is to remove cells and dead cells and other substances; Centrifuge for the second time at 6°C, 1000g, for 20min, discard the precipitate, and take the supernatant. The purpose of this centrifugation is to remove large cell debris and other substances; The supernatant, the purpose of this centrifug...
Embodiment 3
[0082] Example 3 A preparation method of loaded norcantharidin exosomes and its property characterization
[0083] (1) Extraction of exosomes:
[0084]Exosomes were extracted by ultracentrifugation. Bone marrow mesenchymal stem cells (BMSCs) were expanded and cultured in a culture dish. When the cell density reached 80%, the original culture medium containing serum was discarded, washed with PBS three times, added DMEM, and starved for 50 h. Collect the DMEM containing exosomes, centrifuge three times at low temperature to remove macromolecular substances, centrifuge for the first time at 2°C, 200g, centrifuge for 20min, absorb the supernatant, discard the sediment, the purpose is to remove substances such as cells and dead cells; Centrifuge for the second time at 2°C, 3000g, for 10min, discard the precipitate, and take the supernatant. The purpose of this centrifugation is to remove large cell debris and other substances; The supernatant, the purpose of this centrifugation ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Caliber | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com