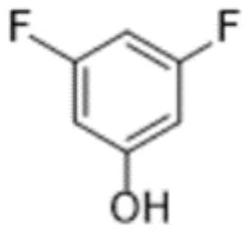
Preparation method of 3, 5-difluorophenol
A technology of difluorophenol and difluorobromobenzene is applied in the field of preparation of compound 3,5-difluorophenol, can solve problems such as few synthetic routes, and achieve the effects of short steps and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
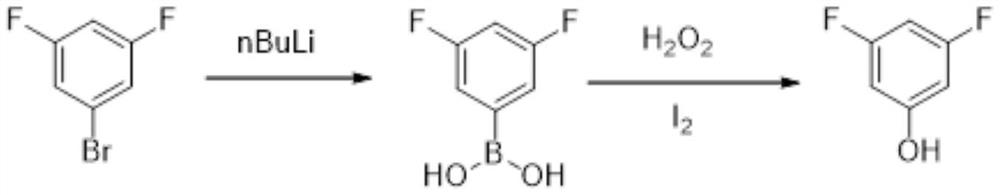
[0030] Its preparation method comprises the following steps:
[0031] Q1. Synthesis of 3,5-difluorophenylboronic acid: 3,5-difluorobromobenzene is used as raw material, and under the protection of an inert gas, a bromine extraction agent is added dropwise to extract bromine, and then reacted with boric acid to obtain 3,5-difluorobenzene boric acid.
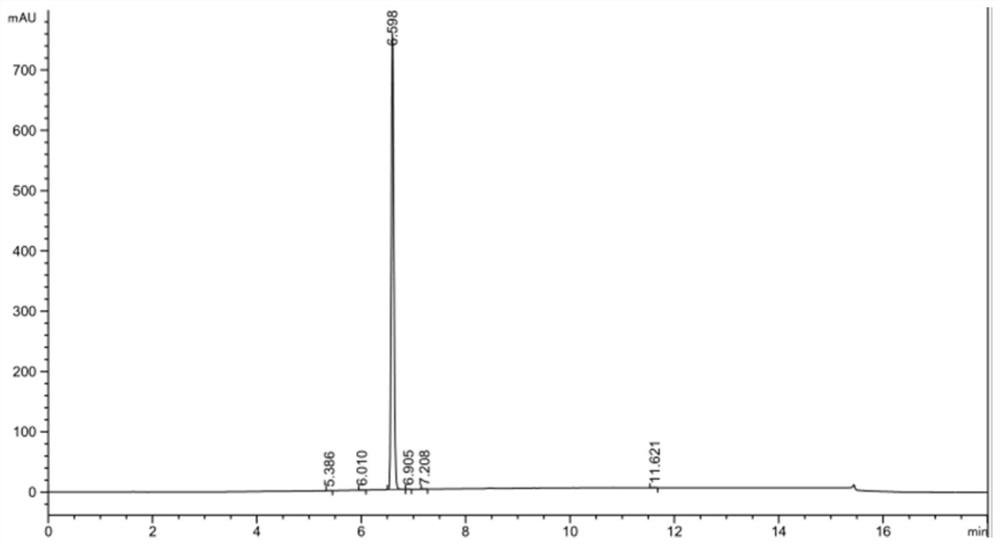
[0032] Q2. Synthesis of 3,5-difluorophenol: Under suitable solvent conditions, 3,5-difluorophenylboronic acid is oxidized by an oxidant and catalyzed by iodine to obtain the final product 3,5-difluorophenol.
Embodiment 1
[0035] Synthesis of 3,5-difluorophenylboronic acid
[0036] Under nitrogen protection, 193g of 3,5-difluorobromobenzene was added to a 2L dry three-necked flask, and then 1000ml of anhydrous tetrahydrofuran was added as a solvent, the temperature was lowered to -70°C, and 320g of n-butyl Lithium, after the dropwise addition is completed, keep warm for 2 hours, then add 92g boric acid and keep warm for 1h, slowly rise to room temperature, and use TLC to determine the reaction end point. After the reaction was completed, filter and dry under reduced pressure to remove the residual solvent to obtain 124.1 g of 3,5-difluorophenylboronic acid with a yield of 78.5%.
Embodiment 2
[0038] Synthesis of 3,5-difluorophenylboronic acid
[0039] Under nitrogen protection, 193g of 3,5-difluorobromobenzene was added to a 2L dry three-necked flask, and then 1000ml of anhydrous tetrahydrofuran was added as a solvent, the temperature was lowered to -60°C, and 320g of n-butyl Lithium, after the dropwise addition is completed, keep warm for 2 hours, then add 92g boric acid and keep warm for 1h, slowly rise to room temperature, and use TLC to determine the reaction end point. After the reaction was completed, it was filtered and dried under reduced pressure to remove the residual solvent to obtain 135.6 g of 3,5-difluorophenylboronic acid with a yield of 85.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com