Preparation method of 1-formyl carbazole
A formyl carbazole and dimethyl formamide technology, applied in the field of organic chemical synthesis, can solve problems such as time-consuming and laborious, high cost of raw materials, and long steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0046] The invention provides a kind of preparation method of 1-formylcarbazole, it comprises the following steps at least:
[0047] (1) In the first anhydrous solvent, the compound of formula (I) is mixed with a strong base, and then reacted with 2-(trimethylsilyl) ethoxymethyl chloride under suitable reaction conditions to generate the first reaction product;
[0048]
[0049] Among them, R 1 and R 2 Each independently represents hydrogen, straight-chain or branched-chain C 1 -C 20Alkyl, aryl or nitro; X represents a halogen atom; and
[0050] (2) react the first reaction product with n-butyllithium and N,N-dimethylformamide sequentially in a second anhydrous solvent, then quench, and remove the protecting group with a removing agent to obtain Second reaction product.
[0051] [step 1]
[0052] Step (1) of the present invention comprises mixing the compound of formula (I) with a strong base in the first anhydrous solvent, and then carrying out the reaction with 2...
Embodiment 1
[0079] The raw materials, solvents and catalysts used in the following examples or comparative examples of the present invention are all conventional commercially available products, unless otherwise specified.
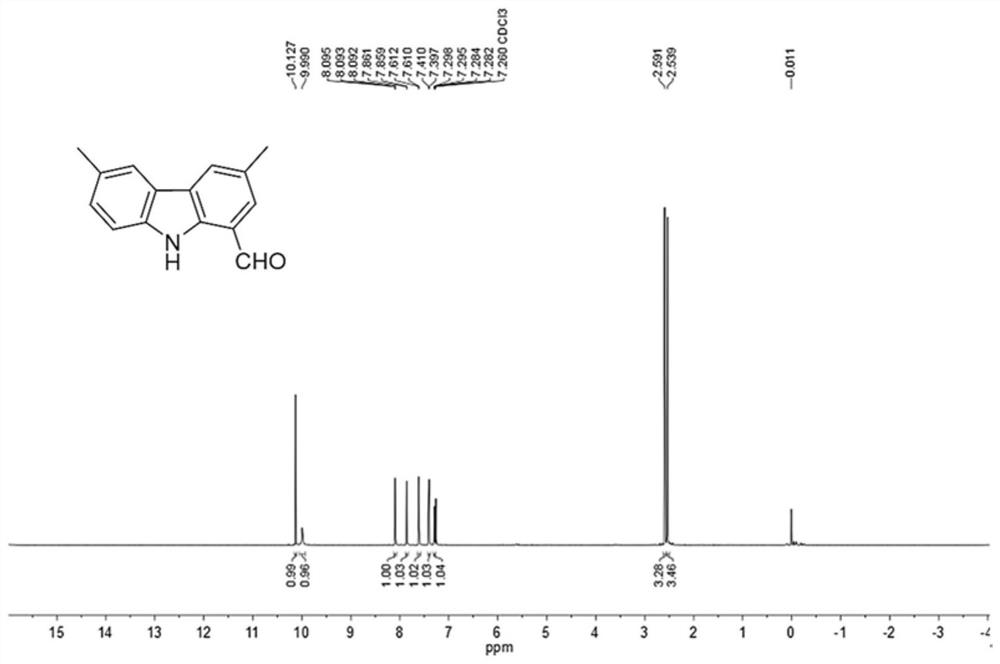
[0080] This embodiment mentions the preparation method of 3,6-dimethyl-1-formylcarbazole, specifically, including the following steps:
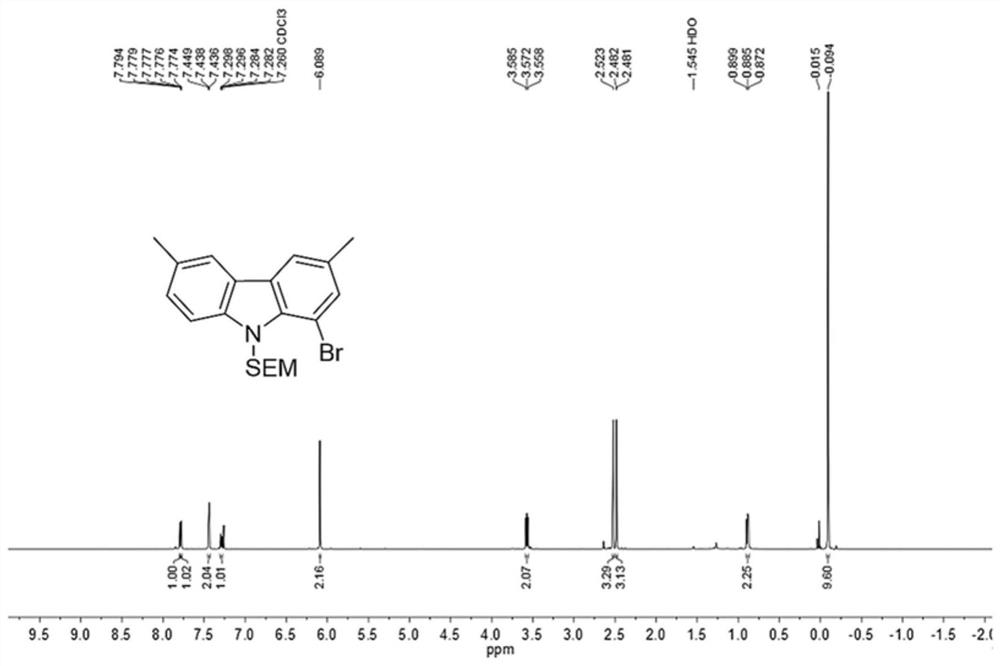
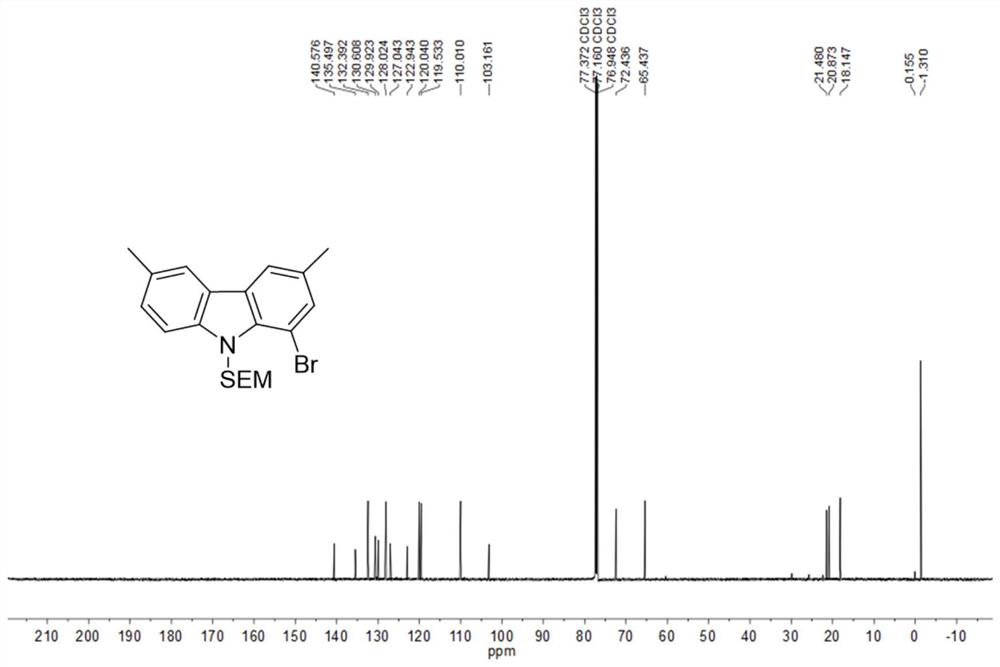
[0081] (1) In a 250mL dry three-necked flask, dissolve 3,6-dimethyl-1-bromocarbazole (5.48g, 20.0mmol) in anhydrous DMF (80mL), and slowly add 60 %NaH (880mg, 22mmol), end the nitrogen flow protection, continue to stir for 0.5h, slowly add SEMCl (3.67g, 22mmol) dissolved in anhydrous DMF (50mL) dropwise, continue stirring for 1.5h after adding, add 10% carbonic acid Extract with sodium hydrogen aqueous solution and DCM, wash the organic phase with water, dry over anhydrous magnesium sulfate, filter, concentrate under reduced pressure, and separate by silica gel column chromatography (developing solvent: DCM:PE=1:1) to obtain the inte...
Embodiment 2
[0084] This embodiment is a preparation method of 3,6-di-tert-butyl-1-formylcarbazole, specifically, the method comprises the following steps:
[0085] (1) In a 250mL dry three-necked flask, dissolve 3,6-di-tert-butyl-1-bromocarbazole (7.17g, 20.0mmol) in anhydrous DMF (80mL), and slowly add in batches under the protection of nitrogen flow 60% NaH (880mg, 22mmol), end the nitrogen flow protection, continue to stir for 0.5h, slowly add SEMCl (3.67g, 22mmol) dissolved in anhydrous DMF (50mL) dropwise, continue to stir for 1.5h, add 10% Sodium bicarbonate aqueous solution and DCM extraction, the organic phase was washed with water, dried over anhydrous magnesium sulfate, filtered, concentrated under reduced pressure, and separated by silica gel column chromatography (developing solvent: DCM:PE=1:1) to obtain the intermediate N-SEM protection 3,6-di-tert-butyl-1-bromocarbazole with a yield of 90%. The obtained intermediate NMR spectrum is as follows Figure 5 Shown, the obtained...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com