Synthesis method of silver(I) trifluoromethanethiolate
A trifluoromethane and synthesis method technology, which is applied in mercaptan preparation, organic chemistry, bulk chemical production, etc., can solve the problems of low utilization rate of silver atoms, high synthesis cost, and many wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022]Example 1: In this example, silver fluoride (3a) (S- (Trifluoromethyl) [1,1'-Biphenyl] -4-Carbothioate) is used to synthesize trifluoride using fluorinated silver. Methane sil silver (I)
[0023]The reaction equation is:
[0024]
[0025]The synthesis steps and processes are: adding fluorinated silver (0.4 mmol, 51 mg) to 10 ml of reaction tubes equipped with magnetic stirrer, and triflicate 3a (1.0 mmol, 282 mg) of phenylbenzoate, and then 4.0 ml of dimethyl sulfoxide was added, and the reaction tube was immobilized on a magnetic stirrer, reacted at a 35 ° C oil bath for 24 hours, and purified to give a target product trifluoromethane silver (I), yield 67.8%.
Embodiment 2
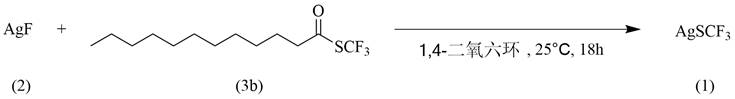
[0026]Example 2: In this example, silver-soluble ferromethane silver (I) is reacted with ferride andtes trifluorometherane (3b) (S- (Trifluoromethyl) DodecaneThioate)
[0027]The reaction equation is:
[0028]
[0029]The synthesis steps and processes are: adding fluorinated silver (0.5 mmol, 63.5 mg), tetralate 3b (2.0 mmol, 568 mg), and then add 4.0 ml to the 10 ml reaction tube equipped with magnetic stirrer. 1.4-dioxane; fixing the reaction tube on the magnetic stirrer, at room temperature for 18 hours, separation and purification to obtain a target product trifluoromethane silver (I), yield 47.7%.
Embodiment 3
[0030]Example 3: In this example, silver fluorine thiol silver is synthesized by hydrofluorine and trifluoromethylfluoromethane (3c) (S- (Trifluoromethyl) 4- (Trifluoromethyl) Benzothioate) (I)
[0031]The reaction equation is:
[0032]
[0033]The synthesis steps and processes are: 1 ml, 1 mmol, 127 mg), which is added to a 10 ml reaction tube equipped with a magnetic stirrer, and trifluoromethyltromethane 3c (1.0 mmol, 274 mg) is added to trifluoromethylbenzoate. Further, 3.0 mL of acetonitrile was added, and the reaction tube was fixed on the magnetic stirrer, and after 15 degrees Celsius was completed for 36 hours, the separation was purified to obtain a target product trifluoromethane silver (I), yield 72.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com