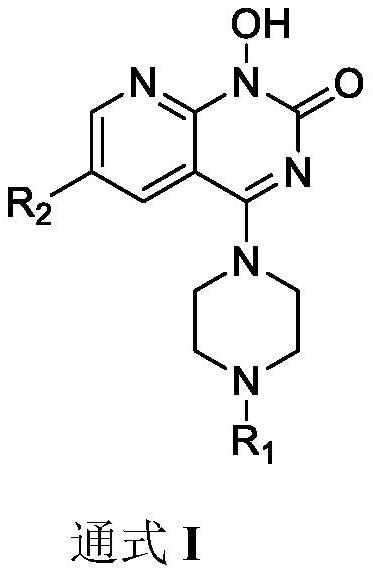
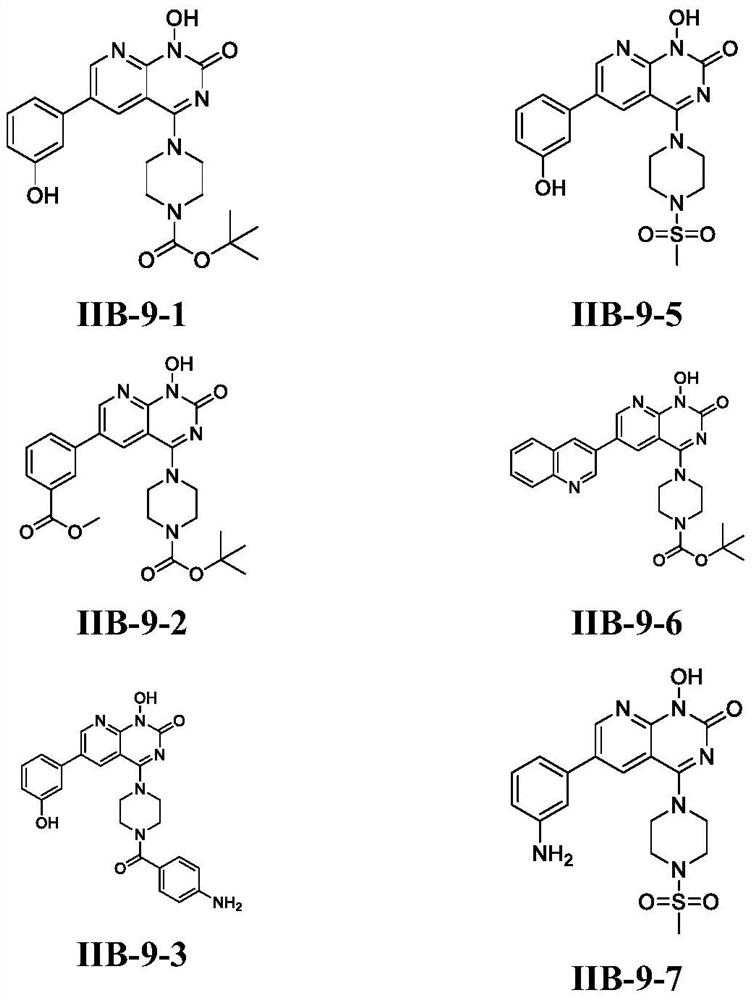
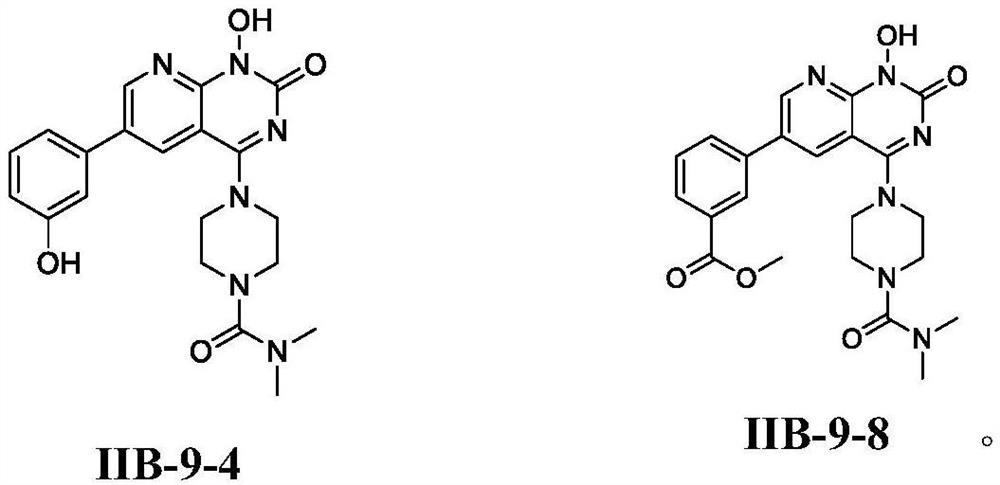
Pyridopyrimidinone compounds and their preparation methods and applications
A compound, pyrimidone technology, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Preparation of 2-((Benzyloxy)amino)-5-bromonicotinic acid methyl ester (intermediate IIB-2)
[0045] 2-Chloro-5-bromonicotinic acid methyl ester IIB-1 (1.0g, 4.0mmol), O-benzyl hydroxylamine (465μL, 4.0mmol) and diisopropylethylamine (2.8mL, 16.0mmol) were weighed together in In a round bottom flask, heat and stir at 60° C. for 6 h (TLC detects the reaction). After the reaction is over, add an appropriate amount of ethyl acetate to the reaction solution for dilution, add dilute hydrochloric acid (20mL*3) to wash, and dry the organic phase over anhydrous sodium sulfate. Column chromatography (eluent is petroleum ether: ethyl acetate = 20 : 1) isolated yellow oil, yield 13%.
Embodiment 2
[0047] Preparation of 1-benzyloxy-6-bromopyrido[2,3-d]pyrimidine-2,4(1H,3H)-dione (intermediate IIB-3)
[0048] Weigh intermediate IIB-2 (0.1g, 0.3mmol) into a 50mL flask, add an appropriate amount of 1,2-dichloroethane, slowly add trichloroacetyl isocyanate (74μL , 0.6mmol) in 1,2-dichloroethane solution, after reacting for half an hour, slowly add triethylamine (86μL, 0.6mmol) in 1,2-dichloroethane solution to the above reaction system, and continue to stir 40min. After the reaction, most of the solvent was evaporated, and the residue was dissolved with an appropriate amount of methanol. Under stirring conditions, 30% w / w methanol solution of sodium methoxide (276 μL, 1.5 mmol) was slowly added to continue stirring for 6 h. After the reaction, most of the solvent was evaporated, redissolved in an appropriate amount of ethyl acetate, the pH of the reaction mixture was adjusted to 3 with 1N hydrochloric acid solution, extracted with ethyl acetate (20 mL*3), and the organic ph...
Embodiment 3
[0050] Weigh compound IIB-3 (1eq) into a round-bottomed flask, add diisopropylethylamine (5eq) and phosphorus oxychloride (5eq) in sequence, heat and stir at 100°C for 45min, then evaporate most of the solvent under reduced pressure to obtain a black Oil (Intermediate IIB-4). Add 10 mL of anhydrous N,N-dimethylformamide to the crude product of IIB-4 above, and then add 1-tert-butoxycarbonylpiperazine (1.5eq) dropwise with anhydrous N,N-dimethylformamide under stirring condition. Base formamide solution, stirred at room temperature for 2h. After the reaction was completed, the reaction solution was poured into ice water and extracted three times with ethyl acetate. The organic phase was successively washed with saturated sodium chloride solution, dried over anhydrous sodium sulfate, and subjected to silica gel column chromatography (volume ratio of petroleum ether: ethyl acetate = 4:1 to 1:1) to obtain a crude product, which was mixed with ethyl acetate / dichloro Recrystalliza...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com