Sirolimus sustained-release microsphere for injection and preparation method of sirolimus sustained-release microsphere
A technology of slow-release microspheres and sirolimus, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, and can solve the problem of low encapsulation efficiency and failure to meet the requirements of the Pharmacopoeia, etc. Problems, to achieve the effect of suitable particle size, avoiding adverse drug reactions, and improving drug bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
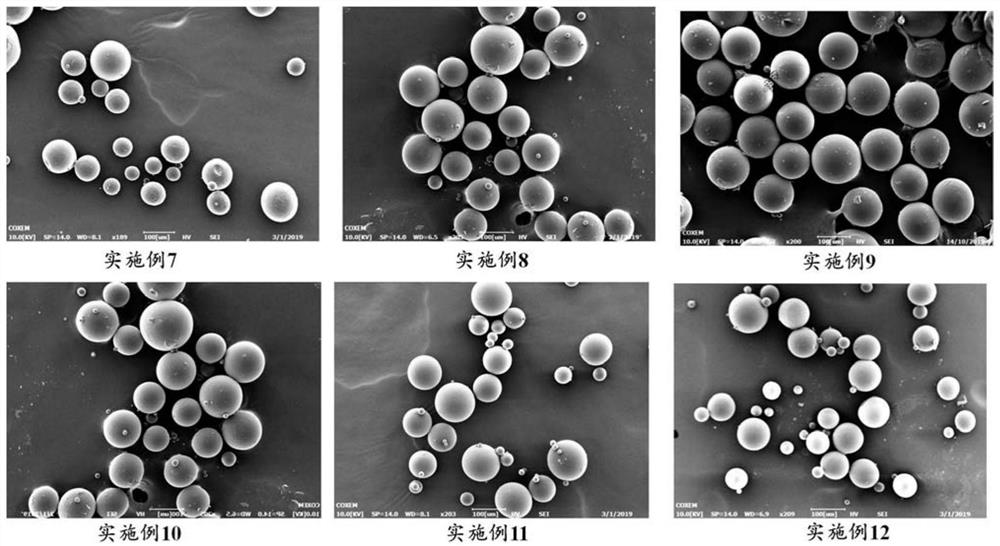
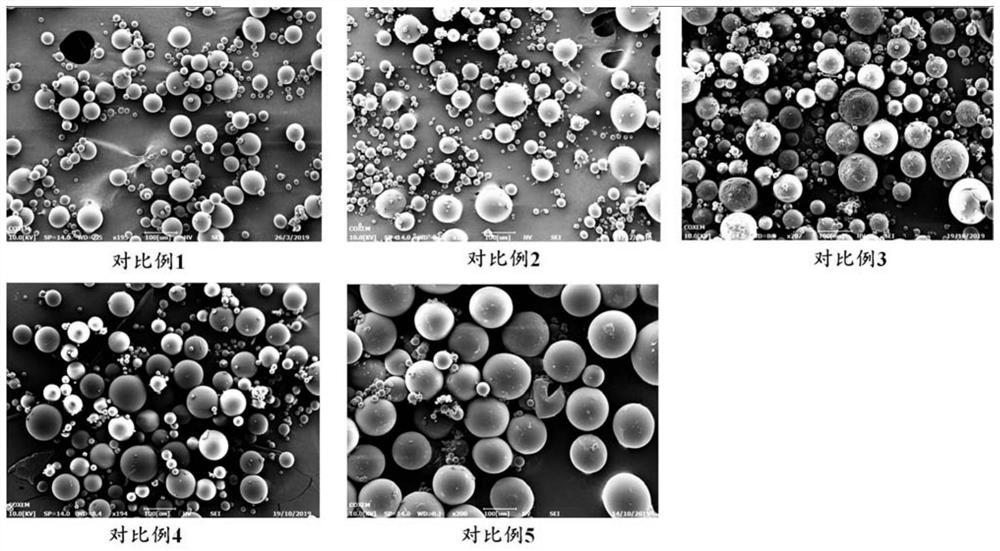
Examples
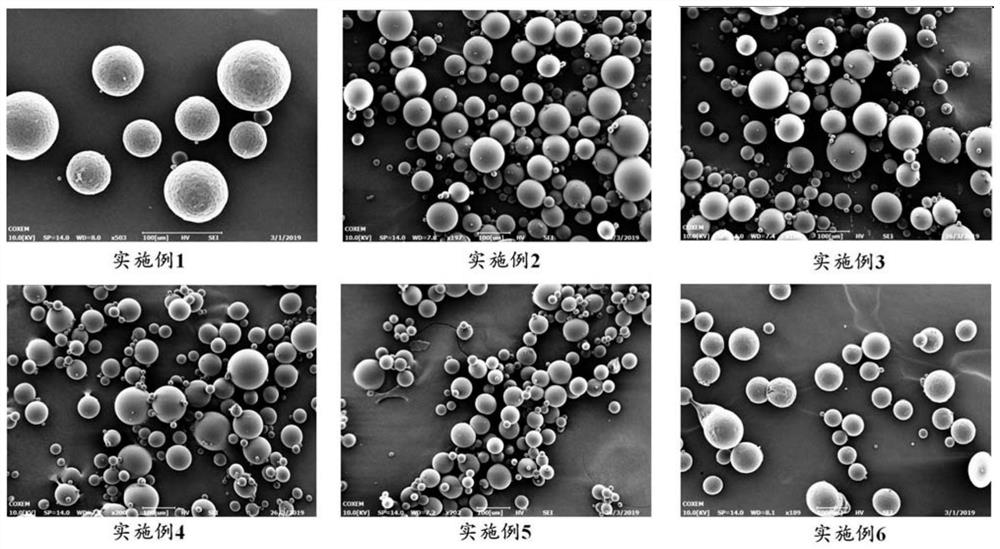
Embodiment 1
[0050] prescription:
[0051]
[0052] Preparation steps:
[0053] 1) Weigh the prescribed amount of sirolimus and PLGA, add to the mixed solvent system (8ml) of propylene carbonate and ethyl acetate, stir and dissolve, and prepare solution 1;
[0054]2) Weigh the prescribed amount of PVA into 500ml of water for injection (100°C), stir until dissolved, cool the PVA solution to 25°C, add water for injection to 800ml, and make a 3mg / mL PVA solution;
[0055] 3) Slowly add solution 1 to 3mg / mL PVA solution, homogeneously emulsify at 1500rpm for 1min;
[0056] 4) After the emulsification is over, turn on the mechanical stirring to evaporate the solvent, and stop stirring after 3 hours;
[0057] 5) Filter the obtained microsphere suspension through a 120-mesh sieve to collect the microspheres, rinse the microspheres with water for injection 3 times, transfer to a petri dish, and freeze-dry in a freeze dryer;
[0058] 6) The freeze-dried product is sieved through a 120-mesh si...
Embodiment 2
[0064] prescription:
[0065]
[0066]
[0067] Preparation steps:
[0068] 1) Weigh the prescribed amount of sirolimus and PLGA, add to the mixed solvent system (8ml) of isopropanol and dichloromethane, stir and dissolve, and prepare solution 1;
[0069] 2) Weigh the prescribed amount of PVA into 500ml of water for injection (100°C), stir until dissolved, cool the PVA solution to 25°C, add water for injection to 800ml, and make a 30mg / mL PVA solution;
[0070] 3) Slowly add solution 1 to 30mg / mL PVA solution, homogeneously emulsify at 1500rpm for 1min;
[0071] 4) After the emulsification is over, turn on the mechanical stirring to evaporate the solvent, and stop stirring after 3 hours;
[0072] 5) Filter the obtained microsphere suspension through a 120-mesh sieve to collect the microspheres, rinse the microspheres with water for injection 3 times, transfer to a petri dish, and freeze-dry in a freeze dryer;
[0073] 6) The freeze-dried product is sieved through a 12...
Embodiment 3
[0079] prescription:
[0080]
[0081] Preparation steps:
[0082] 1) Weigh the prescribed amount of sirolimus and PLGA, add to the mixed solvent system (8ml) of benzyl alcohol and dichloromethane, stir and dissolve, and prepare solution 1;
[0083] 2) Weigh the prescribed amount of PVA and add it to 500ml water for injection (100°C), stir until dissolved, cool the PVA solution to 25°C, add water for injection to 800ml, and make 8mg / mL PVA solution;
[0084] 3) Slowly add solution 1 to 8mg / mL PVA solution, homogeneously emulsify at 1500rpm for 1min;
[0085] 4) After the emulsification is over, turn on the mechanical stirring to evaporate the solvent, and stop stirring after 3 hours;
[0086] 5) Filter the obtained microsphere suspension through a 120-mesh sieve to collect the microspheres, rinse the microspheres with water for injection 3 times, transfer to a petri dish, and freeze-dry in a freeze dryer;
[0087] 6) The freeze-dried product is sieved through a 120-mesh ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com