ProA/G-dRep fusion protein serving as universal carrier of nucleic acid-antibody codons and application of ProA/G-dRep fusion protein
A fusion protein and antibody technology, applied in the field of genetic engineering, can solve the problems of uncontrollable coupling quantity, uncontrollable coupling quantity, antibody inactivation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] 1. Selection of the amino acid sequence of the ProA / G-dRep fusion protein gene:
[0046] The amino acid sequence of duck circovirus replicase (Duck circovirus Rep) (Uniprot Accession No.A7LI84, SEQ ID No.4) is as follows:
[0047] MAKSGNYSYKRWVFTINNPTFEDYVHVLEFCTLDNCKFAIVGEEKGANGTPHLQGFLNLRSNARAAALEESLGGRAWLSRARGSDEDNEEYCAKESTYLRVGEPVSKGRSSDLAEATSAV;
[0048] The fusion protein (ProA / G) amino acid sequence (SEQ ID No.2) of protein A / G is as follows:
[0049] VDNKFNKEQQNAFYEILHLPNLNEEQRNAFIQSLKDDPSQSANLLAEAKKLNDAQAPK;
[0050] The amino acid sequence (SEQ ID No.3) of flexible peptides is as follows:
[0051] GGGGSGGGGSGGGGS;
[0052] 2. ProA / G-dRep fusion protein amino acid sequence design
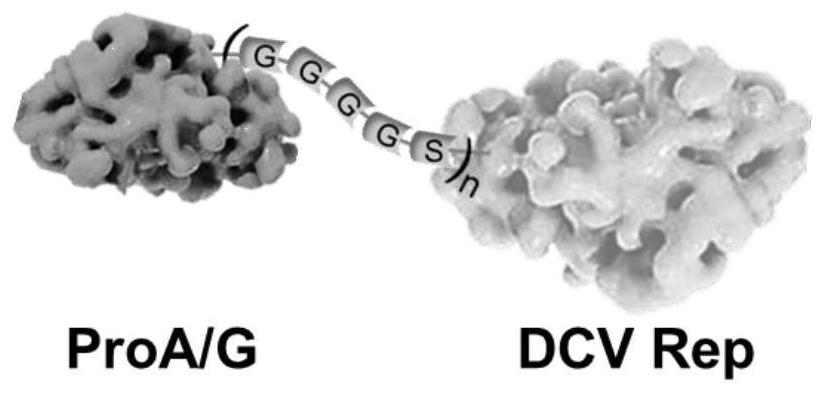
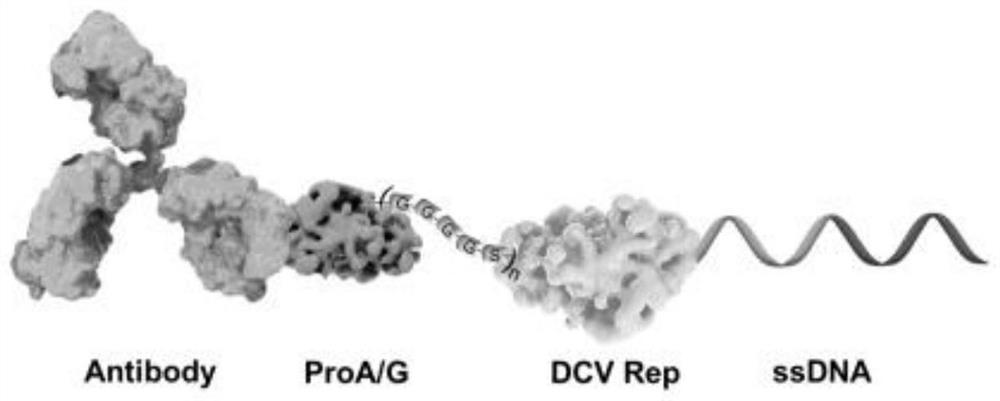
[0053] Place ProA / G at the N-terminus of the ProA / G-dRep fusion protein, place the flexible peptide between ProA / G and dRep, and place dRep at the C-terminus of the ProA / G-dRep fusion protein, and the result is as follows figure 1 As shown, the amino acid sequence is the ProA / G-...
Embodiment 2
[0059] 1. Preparation of ProA / G-dRep fusion protein genetic engineering bacteria
[0060] (1) Take 2 μL of the dissolved plasmid and add it to BL21(DE3) competent cells, and ice-bath for 10 minutes;
[0061] (2) After being heat-shocked in a water bath at 42°C for 90 seconds, immediately place it in an ice bath for 5 minutes;
[0062] (3) Add 900 μL of anti-LB medium, fix it horizontally in a shaker, shake at 37°C for 60 min;
[0063] (4) Discard 800 μL of the supernatant by centrifugation, resuspend the remaining bacterial solution and spread evenly on the LB plate containing 50 μg / ml kanamycin;
[0064] (5) Place it upside down in a constant temperature incubator and culture at 37°C for 12 hours to obtain the ProA / G-dRep fusion protein genetically engineered bacterium BL21(DE3) / pET-28a-ProA / G-dRep.
[0065] 2. Fermentation expression
[0066] (1) Take the well-grown monoclonal cells from the plate and transfer them to a test tube containing 5 mL of LB liquid medium (50 μg...
Embodiment 3
[0090] Ligation test of single-stranded deoxyribonucleic acid and ProA / G-dRep fusion protein
[0091] 1. Preparation of single-stranded deoxyribonucleic acid
[0092] (1) According to the literature (J.Am.Chem.Soc.2017, 139, 7030-7035), the specific single-stranded DNA sequence recognized by dRep is: 5'-AAG TA TTACCAGAAA-3' (SEQ ID No.5, referred to as dRep ssDNA for short, the underline represents dRep specifically recognizes and cleaves and connects the single-stranded deoxyribonucleic acid site, the molecular weight is about 4.655kD);
[0093] (2) Send the above deoxyribonucleic acid sequence to Shanghai Sangong for synthesis, add 100mM NaCl solution after synthesis to dissolve to a concentration of 100μM, and freeze at -30°C for later use.
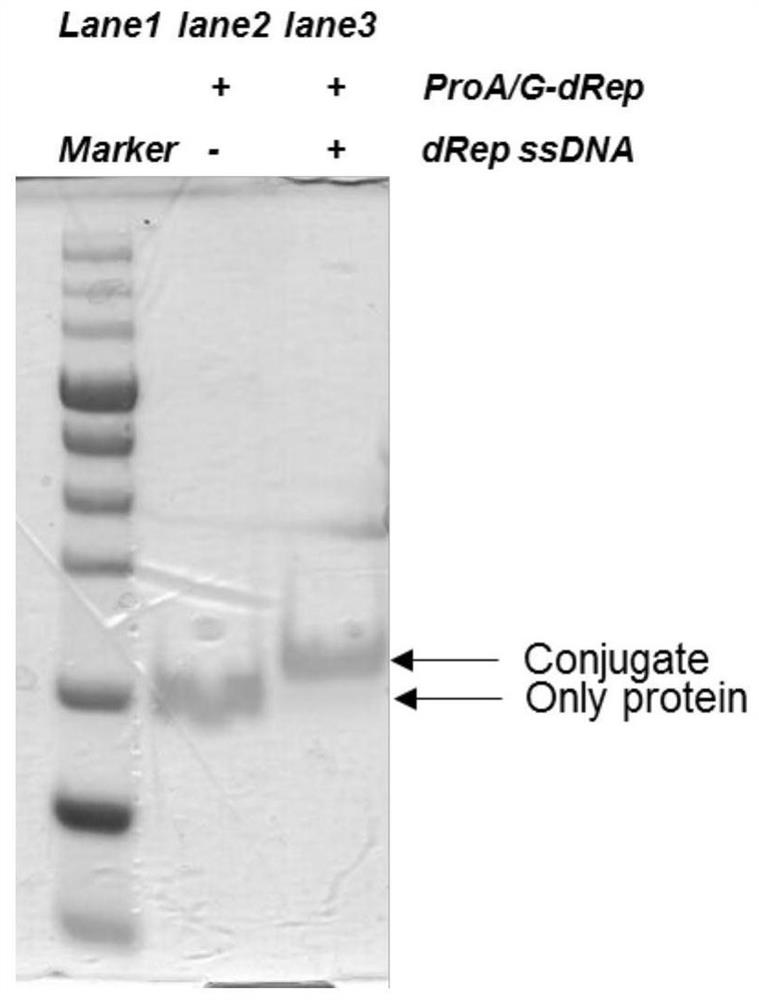
[0094] 2. Single-stranded DNA ligation test
[0095] (1) Take ProA / G-dRep solution and dRep ssDNA to carry out the ligation reaction according to the table below, and the ligation reaction system is shown in Table 1:
[0096] Table...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com