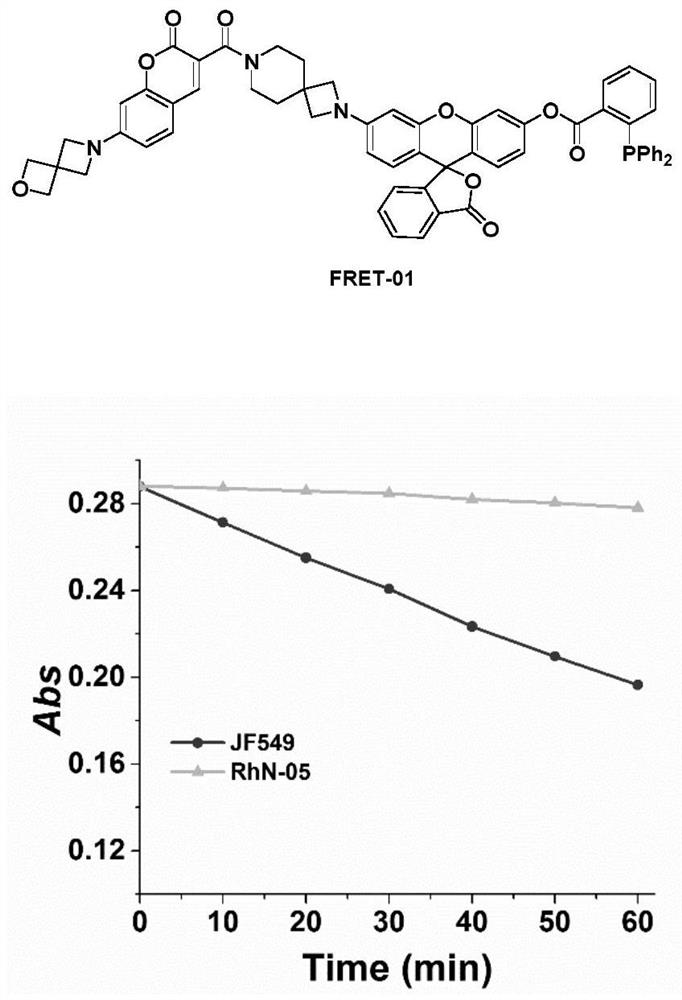
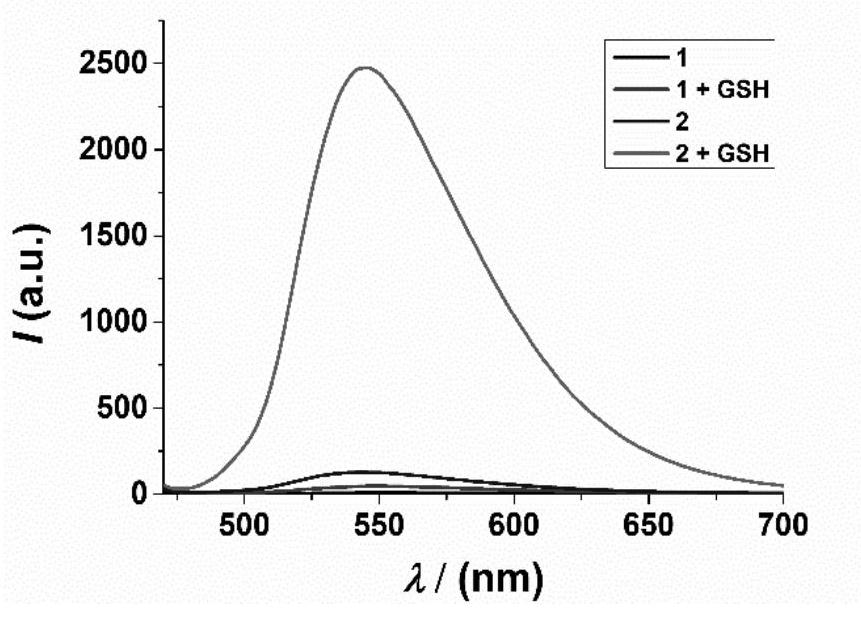
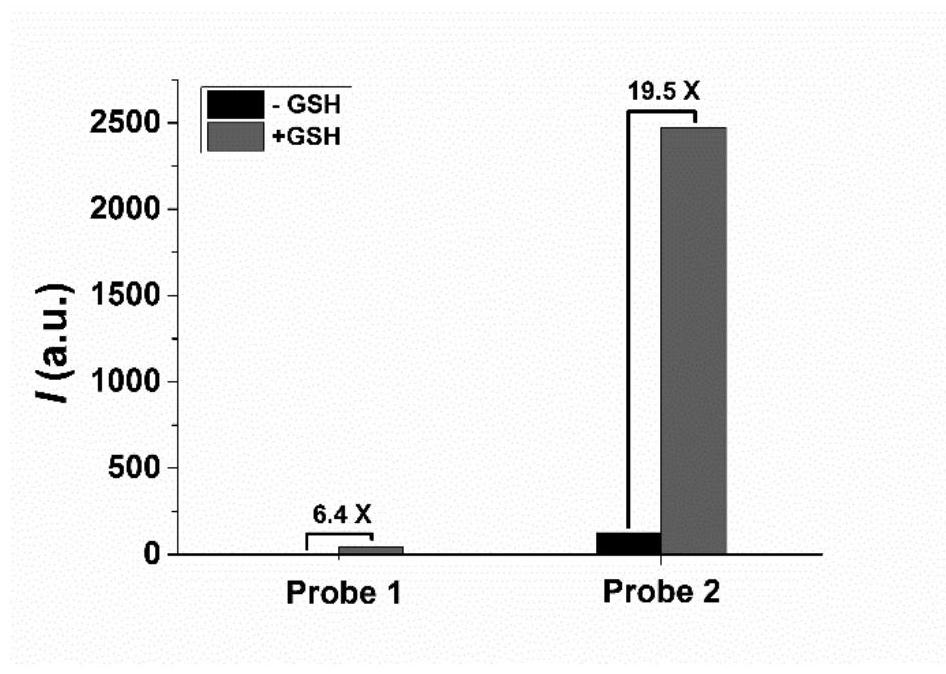
Fluorescent dye containing azetidine spiro structure, and preparation method and application thereof
An azetidine and spiro structure technology, applied in the field of fluorescence analysis, can solve the problems of lack of brightness and photostability of single-molecule and live cell imaging, low photostability of fluorescent dyes, and no reports.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0128] Embodiment 1 prepares compound Nap-02
[0129]
[0130] Nap-00 (100mg, 0.3mmol) and azetidine hydrochloride (94mg, 1.0mmol) were dissolved in triethylamine (0.2mL) and DMSO (3mL), the reaction was heated to 120°C overnight, LC- MS detected that the reaction was complete, and the reaction solution was purified by reverse-phase preparative chromatography to obtain the product Nap-02 (59 mg, yield: 64%). 1 H NMR (400MHz, DMSO-d 6 )δ=8.39(dd, J=1.0,7.3Hz,1H),8.34(dd,J=1.0,8.4Hz,1H),8.18(d,J=8.6Hz,1H),7.58(dd,J=7.3 ,8.4Hz,1H),6.43(d,J=8.6Hz,1H),4.47(t,J=7.5Hz,4H),4.04-3.96(m,2H),2.50-2.42(m,2H),1.63 -1.53(m,2H),1.39-1.29(m,2H),0.92(t,J=7.3Hz,3H). 13 C NMR (101MHz, DMSO-d 6 )δ=164.11, 163.27, 152.70, 133.23, 131.19, 131.12, 130.33, 124.32, 122.12, 120.59, 108.73, 106.50, 55.59, 30.25, 20.31, 16.94, 14.21. MS (ESIC): calc' 19 h 20 N 2 o 2 [M+H] + 309.2, measured 309.0; HRMS (ESI): calc'd for [M+H] + 309.15975, measured 309.16024.
Embodiment 2
[0131] Embodiment 2 prepares compound Nap-03
[0132]
[0133] Nap-00 (100mg, 0.3mmol) and 2-oxa-6-aza-spiro[3,3]heptane oxalate (72mg, 0.5mmol) were dissolved in triethylamine (0.2mL) and DMSO ( 3 mL), the reaction was heated to 120° C. overnight, LC-MS detected that the reaction was complete, and the reaction solution was purified by reverse-phase preparative chromatography to obtain the product Nap-03 (49 mg, yield: 47%). 1 H NMR (400MHz, DMSO-d 6 )δ=8.40(ddd,J=0.9,7.9,18.0Hz,2H),8.23(d,J=8.4Hz,1H),7.64(dd,J=7.4,8.4Hz,1H),6.53(d,J =8.6Hz,1H),4.79(s,4H),4.67(s,4H),4.07-3.97(m,2H),2.04-1.94(m,1H),1.67-1.51(m,2H),1.39- 1.29(m,2H),0.91(t,J=7.3Hz,3H). 13 C NMR (101MHz, DMSO-d 6 )δ=164.12,163.32,152.47,133.23,131.26,131.06,130.27,130.10,124.62,122.27,120.88,109.38,107.22,80.14,64.47,39.47,39.12,35.58,30.24,29.49,29.19,29.14,29.04,25.57 ,20.30,14.41,14.21.MS(ESI):calc'd for C 21 h 22 N 2 o 3 [M+H] + 351.2, measured 351.0; HRMS (ESI): calc'd for [M+H] + 351.17032, mea...
Embodiment 3
[0134] Embodiment 3 prepares compound Nap-04
[0135]
[0136] Nap-00 (100mg, 0.3mmol) and 6-oxa-1-aza-spiro[3,3]heptane oxalate (72mg, 0.5mmol) were dissolved in triethylamine (0.2mL) and DMSO ( 3 mL), the reaction was heated to 120° C. overnight, LC-MS detected that the reaction was complete, and the reaction solution was purified by reverse-phase preparative chromatography to obtain the product Nap-04 (45 mg, yield: 43%). 1 H NMR (400MHz, Methanol-d 4 )δ=8.60(d, J=8.4Hz, 1H), 8.50(d, J=7.3Hz, 1H), 8.38(d, J=8.7Hz, 1H), 7.59(dd, J=7.5, 8.6Hz, 1H), 7.49(d, J=8.7Hz, 1H), 5.47(d, J=8.9Hz, 2H), 4.70(t, J=7.3Hz, 2H), 4.17-4.05(m, 2H), 3.35( s,2H),2.76(t,J=7.3Hz,2H),1.75-1.64(m,2H),1.50-1.40(m,2H),1.01-0.94(m,3H). 13 C NMR (101MHz, Methanol-d 4 )δ=164.75,164.17,150.02,137.22,133.09,131.07,131.00,130.50,123.59,122.01,121.27,108.89,106.57,79.34,73.82,52.73,39.46,29.92,28.35,19.96,12.80.MS(ESI): calc'd for C 21 h 22 N 2 o 3 [M+H] + 351.2, measured 351.1; HRMS (ESI): calc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com