Vonoprazan salt as well as preparation method and application thereof
A technology of complexes and organic acids, applied in the field of salts of pyrrole compounds and salts formed by complexes, can solve the problems of acid rebound, research on biological activity and its bioavailability, influence on therapeutic effects, etc. Improved availability, good solubility and stability, and increased efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] This example prepares the salt formed by the complex of vonoprazan, citric acid and bismuth.
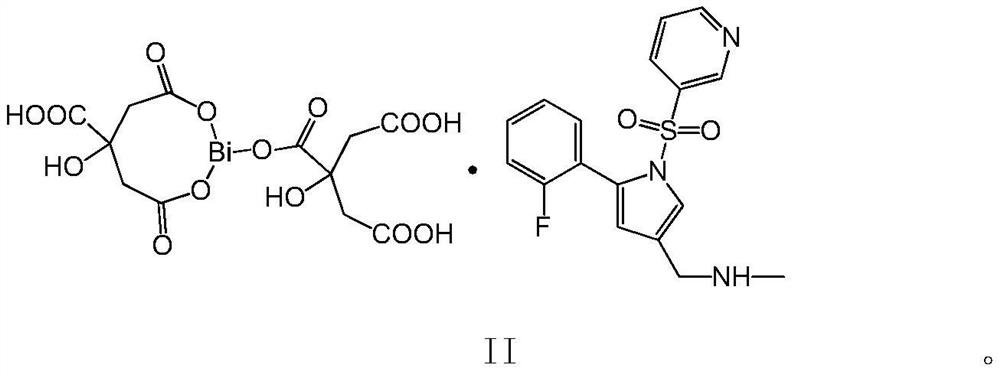
[0052] 1. The chemical formula of the salt formed by the complex of Vonorazan with citric acid and bismuth is C 29 h 29 BiFN 3 o 16 S, molecular weight is 935.59; Its molecular structure is as shown in formula II below:
[0053]
[0054] 2. The preparation method of the salt formed by the complex of Vonorazan and citric acid and bismuth in this embodiment, its synthetic route is as follows:
[0055]
[0056] Specifically include the following steps:
[0057] (1) Vonorazan can be obtained through commercial channels or synthesized through existing technologies.
[0058] Compound IX is the fumarate salt of Vonorazan. DCM is dichloromethane.
[0059] The synthetic method of compound I Vonorazan in the present embodiment is as follows:
[0060] Compound IX (4.0g, 8.67mmol, 1.0eq) was added to a mixture of 20mL water and 20mL DCM, keeping the temperature at 25°C, and ...
Embodiment 2
[0070] This example prepares the salt formed by the complex of vonorazan, tartaric acid and bismuth.
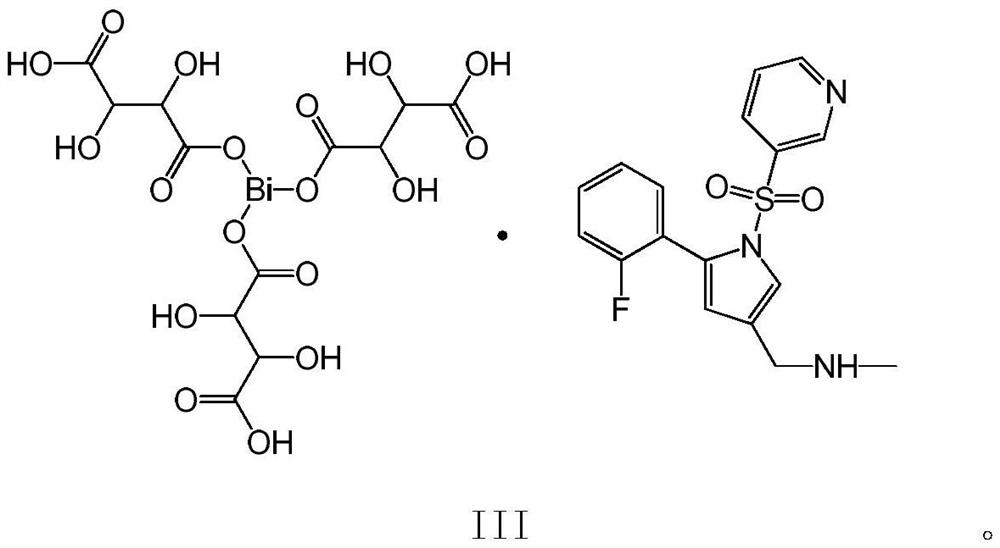
[0071] 1. The chemical formula of the salt formed by the complex of Vonorazan, tartaric acid and bismuth is C 29 h 31 BiFN 3 o 20 S, molecular weight is 1001.61; Its molecular structure is as shown in formula III below:
[0072]
[0073] 2. The preparation method of the salt formed by the complex of Vonorazan and tartaric acid and bismuth of the present embodiment, its synthetic route is as follows:
[0074]
[0075] Specifically include the following steps:
[0076] (1) Synthesis of Compound VII Bismuth Potassium Tartrate:
[0077] Bismuth nitrate (6.0g, 15.2mmol, 1.0eq) was added to 30mL water, stirred for 30min, 6.16g of 25wt% KOH aqueous solution was added dropwise, the pH was measured to be 2, and the stirring was continued for 30min, and a white solid was obtained by suction filtration, which was added to In 20mL water, add dropwise the KOH aqueous solution ...
Embodiment 3
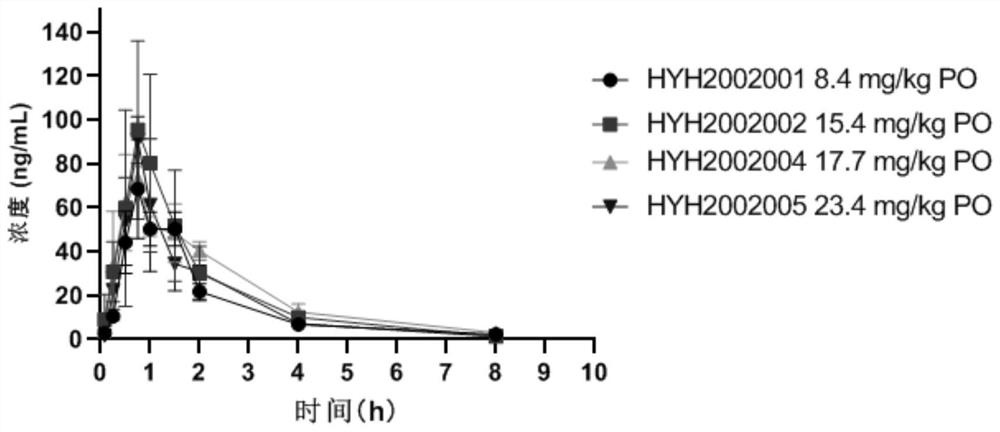
[0085] Bioactive Pharmacokinetic Testing
[0086] Experimental test drugs: HYH2002001 (control group 1, commercially available vonoprazan fumarate), HYH2002002 (example 1, the salt formed by the complex of vonoprazan, citric acid and bismuth), HYH2002004 ( Example 2, the salt formed by the complex of vonoprazan, tartaric acid and bismuth), HYH2002005 (control group 2, commercially available bismuth potassium citrate and vonoprazan fumarate according to the molar ratio of 1:1 than physical mixing);
[0087] Experimental method: 60 male SD rats (200-250g) were randomly divided into four groups, and HYH2002001 (8.4g kg-1), HYH2002002 (15.4g kg-1), HYH2002004 (17.7g kg -1), HYH2002005 (23.4g·kg-1), wherein HYH2002001, HYH2002002, HYH2002004, and HYH2002005 are administered with equimolar vonoprazan, and the administration volume is 5ml·kg-1. Blood was collected at 5min, 15min, 30min, 45min, 1h, 1.5h, 2h, 4h, and 8h after administration, and plasma was collected by centrifugation...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com