Application of substituted crotonamide
A technology of ethoxy and ethynylbenzene, which is applied in the application field of substituted crotonamides and can solve unrecorded problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The IC50 determination of embodiment 1 protein kinase
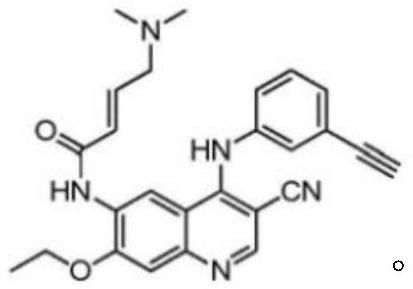
[0034] The compound tested in this example is (E)-N-(3-cyano-7-ethoxy-4-(3-ethynylphenylamino)quinolin-6-yl)-4-(dimethyl amino) but-2-enamide.
[0035] 1. Experimental Design
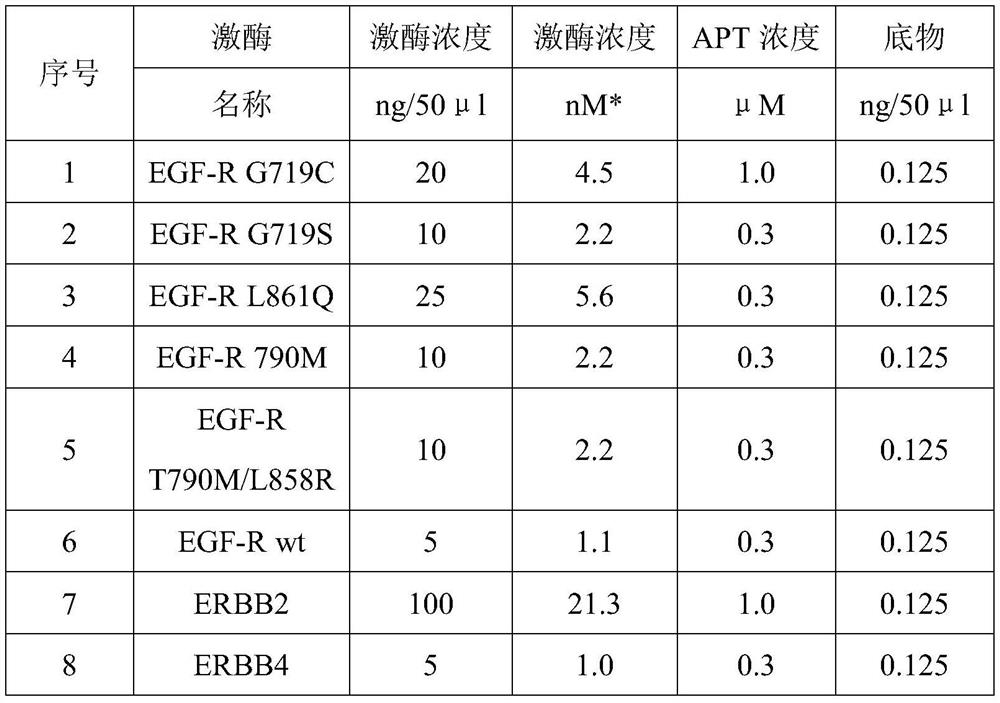
[0036] For the IC50 of the test compound on the protein kinase, 10 semi-logarithmic concentrations (1×10 -06 M to 3×10 - 11 M), single hole, IC50 determination.
[0037] 2. Test substance
[0038] The test substance was two solid tubes, and the ProQinase was transferred to -20°C for subsequent use.
[0039] Before the experiment, make up 1×10 with 100% DMSO -03 M stock solution of test substance and further diluted to 1×10 -04 M / 100% DMSO.
[0040] With 100% DMSO, the test substance was half-logarithmically diluted to a 96-well plate, and prepared from 1 × 10 -04 M to 3×10 -09 M. Before use, the test substance was diluted 1:10 with water to obtain 1×10 -05 M to 3×10 -10 M test substance samples, containing 10% DMSO.
[0041] 5...
Embodiment 2
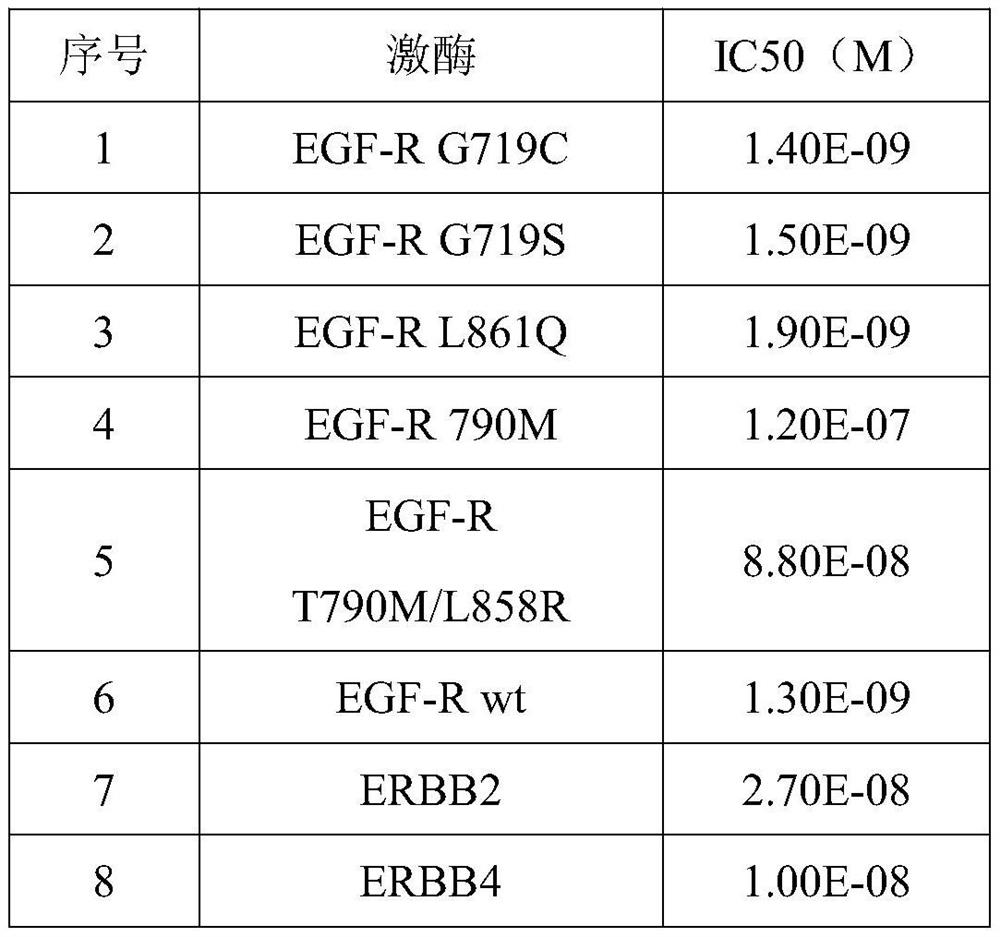
[0063] The above data show that the inhibitory effect of the tested compounds on rare mutations is better than that of general non-rare mutations. Example 2 Exploratory clinical research trials for the treatment of locally advanced or metastatic non-small cell lung cancer
[0064] 1. Subject selection criteria
[0065] 1. Inclusion criteria:
[0066] Age 18-75 (including 18, 75) years old, gender is not limited. Patients with locally advanced or metastatic NSCLC confirmed by histopathology and / or cytology. Expected survival time > 3 months. The patient's EGFR has one or more of L861Q, G719X, and S768I mutations, and does not have T790M primary / de novo mutations, exon 20 insertion mutations, or L858R mutations.
[0067] 2. Exclusion criteria:
[0068] Patients cannot participate in this trial if they meet any of the following conditions:
[0069] (1) Received any epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) anti-tumor therapy before enrollment.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com