1-(7-ethoxycoumarin)-4-(2-methyl-8-methoxyquinoline)-1, 2, 3-triazole ratiometric fluorescence or ratiometric ultraviolet absorption probe agent and preparation and application thereof
A quinoline oxymethyl, ratiometric fluorescence technology, applied in fluorescence/phosphorescence, luminescent materials, chemical instruments and methods, etc., can solve problems such as aluminum ion toxicity, and achieve novel compound structures, high yields, and simple synthesis methods. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment one: compound a That is, the synthesis of 1-(7-coumarinethoxy)-4-(2-methyl-8-quinolineoxymethyl)-1,2,3-triazole:
[0058] In a 100ml three-necked flask under nitrogen protection, add 1.00g (6.29mmol) of 8-hydroxyquinaldine, 1.30g (9.44mmol) of potassium carbonate and 50ml of acetone, reflux for 0.5h, cool, and then add 3-bromopropyne 1.05 g Gray intermediate: 2-methyl-8-(prop-2-ynyloxy)quinoline, yield 85.0%.
[0059] In a 100ml three-necked flask under nitrogen protection, add 2.34g (10.15mmol) of 7-(2-azidoethoxy)-coumarin, 1.00g (5.76mmol) of the above intermediate and cuprous iodide (catalytic amount), reacted at room temperature for 10h, finished, filtered, the filtrate was concentrated to remove the solvent, and purified by column chromatography, the eluent was: ethyl acetate / petroleum ether (1 / 1, v / v) to obtain 1.73g of the white target product a : 1-(7-coumarinethoxy)-4-(2-methyl-8-quinolineoxymethyl)-1,2,3-triazole, yield 70.0%. m.p.141~143℃; 1 ...
Embodiment 2
[0061] The preparation method of various reagents in various analytical methods of the present invention is:
[0062] (1) compound a Compound solution preparation method: weigh 4.3 mg of compound a , dissolved in ethanol to prepare a 100mL solution with a concentration of 100μmol L -1 ;
[0063] (2)Al 3+ Standard solution: weigh analytically pure Al(NO 3 ) 3 9H 2 O 75.0mg, dissolved in twice distilled water, and prepared into 100mL solution, Al 3+ The concentration is 2.00×10 -3 mol L -1 ; Dilute step by step with double distilled water to a suitable concentration as required;
[0064] (3) Preparation of other coexisting ionic solutions: Take analytically pure nitrates or hydrochlorides of various metals, dissolve them in double distilled water, and prepare them at a concentration of 2.00×10 -3 mol L -1 double distilled aqueous solution.
[0065] The used ultraviolet-visible spectrophotometer model of the present invention is UV-1800, and Shimadzu Corporation p...
Embodiment 3
[0068] Add compound to 10.0 mL volumetric flask a ethanol stock solution (1.00 x 10 -4 mol L -1 , 1mL), metal ion Al 3+ (2.00×10 -3 mol L -1, 1 mL), dilute to the mark with ethanol / water (2 / 3, v / v) solution, shake well, transfer to a 1cm quartz cuvette for fluorescence spectrum and ultraviolet absorption spectrum measurement.
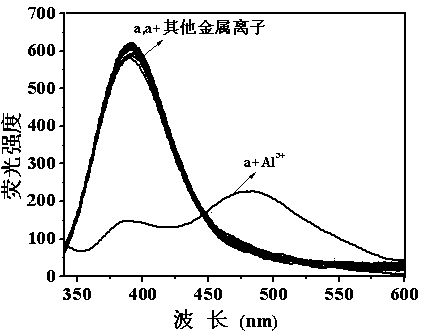
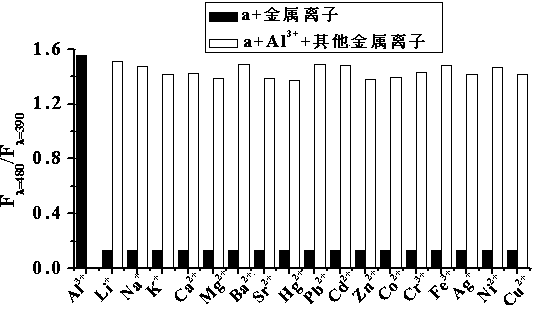
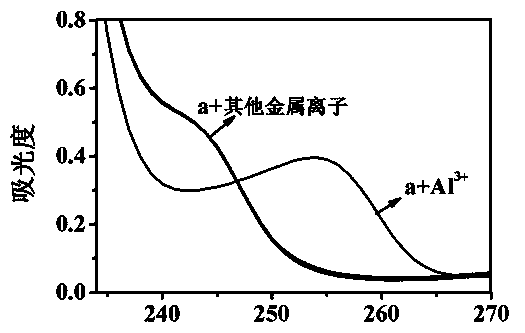
[0069] Set the fluorescence excitation wavelength to 318nm, add about 3ml of compound into a 1cm cuvette a (Concentration is 1.00×10 -5 mol L -1 ) in ethanol / water (2 / 3, v / v) solution for fluorescence spectrum test, compound a There is fluorescence emission at a wavelength of 390 nm. Join Al 3+ (Concentration is 2.00×10 -4 mol L -1 ), the compound a The fluorescence intensity at 390nm of the solution was significantly reduced (quenching rate was 90.46%), and an emission peak appeared at 480nm (fluorescence intensity was 145). The wavelengths are 390nm and 480nm respectively to form a ratio fluorescence, and there is an iso-emission poi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com