Pyridine compounds as inhibitors of dipeptidyl peptidase iv
A compound, amylpyridine technology, applied in the field of pyridine compounds, can solve problems such as no compound report, and achieve the effect of excellent peptidase inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific example
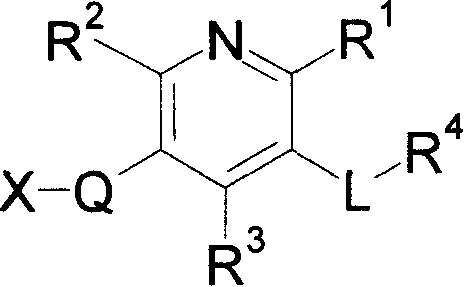
[0226] As examples of the "divalent chain hydrocarbon group" of L or Q, a divalent chain hydrocarbon group having 1 to 10 carbon atoms can be mentioned. Specific examples of this include:
[0227] (1)C 1-10 Alkylene (for example, -CH 2 -, -(CH 2 ) 2 -, -(CH 2 ) 3 -, -(CH 2 ) 4 -, -(CH 2 ) 5 -, -(CH 2 ) 6 -, -CHCH 3 -, -C(CH 3 ) 2 -, -(CH(CH 3 )) 2 -, -(CH 2 ) 2 C(CH 3 ) 2 -, -(CH 2 ) 3 C(CH 3 ) 2 -);
[0228] (2)C 2-10 Alkenylene (for example, -CH=CH-, -CH 2 -CH=CH-, -CH=CH-CH 2 -, -CH=CH-CH 2 -CH 2 -, -C(CH 3 ) 2 -CH=CH-, -CH 2 -CH=CH-CH 2 -, -CH 2 -CH 2 -CH=CH-, -CH=CH-CH=CH-, -CH=CH-CH 2 -CH 2 -CH 2 -);
[0229] (3)C 2-10 Alkynylene (for example, -C=C-, -CH 2 -C=C-, -CH 2 -C=C-CH 2 -CH 2 -);Wait.
[0230] Preferably the "divalent chain hydrocarbon group" is C 1-10 Alkylene or C 2-10 Alkenylene, more preferably it is -CH 2 -, -(CH 2 ) 2 -, -CH=CH- etc.
[0231] L is preferably C 1-10 Alkylene, more preferably -CH 2 -Wait. ...
Embodiment 1
[0691] 5-(Aminomethyl)-6-isobutyl-2-methyl-4-(4-methylphenyl)nicotinic acid methyl ester
[0692] 1) A suspension of sodium hydride (60% in oil, 8.0 g, 0.2 mol) in tetrahydrofuran (80 mL) was heated under reflux with vigorous stirring. To the resulting suspension was added dropwise a mixture of methyl isovalerate (11.6 g, 0.1 mol), acetonitrile (10.5 mL, 0.2 mol) and tetrahydrofuran (25 mL), and the mixture was heated to reflux for 5 hours. The reaction mixture was cooled to room temperature, and 2-propanol (5 mL) was added thereto. The mixture was stirred at room temperature for 30 minutes. The reaction mixture was concentrated under reduced pressure, and the residue was dissolved in water (100 mL), and washed successively with hexane and a hexane-ether mixed solution. The aqueous layer was acidified with concentrated hydrochloric acid and extracted with ether. The extract was washed with water and dried over anhydrous magnesium sulfate. The solvent was evaporated under r...
Embodiment 2
[0702] 5-(aminomethyl)-6-isobutyl-2-methyl-4-(4-methylphenyl)nicotinic acid dihydrochloride
[0703] 1) Dissolve 5-(aminomethyl)-6-isobutyl-2-methyl-4-(4-methylphenyl)nicotinic acid methyl ester (0.90g, 2.76mmol) in tetrahydrofuran (25mL) To the solution, di-tert-butyl dicarbonate (0.76 mL, 3.31 mmol) was added, and the mixture was stirred at room temperature for 12 hrs. The reaction mixture was concentrated under reduced pressure, and the residue was purified by silica gel column chromatography to obtain 5-{[(tert-butoxycarbonyl)amino]methyl}-6-isobutyl-2-methyl-4-(4 -Methylphenyl)nicotinate (1.16 g, yield 98%), which is a white powder.
[0704] 1 H-NMR (CDCl 3 )δ: 0.97 (6H, d, J=6.8Hz), 1.39 (9H, s), 2.10-2.30 (1H, m), 2.39 (3H, s), 2.54 (3H, s), 2.78 (2H, d , J=7.2Hz), 3.50(3H, s), 4.15(2H, d, J=4.9Hz), 4.24(1H, t, J=4.9Hz), 7.06(2H, d, J=7.9Hz), 7.20 (2H, d, J = 7.9 Hz).
[0705]2) To 5-{[(tert-butoxycarbonyl)amino]methyl}-6-isobutyl-2-methyl-4-(4-methylphenyl)nicoti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com