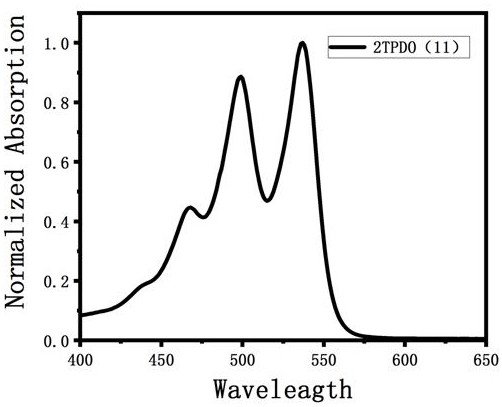
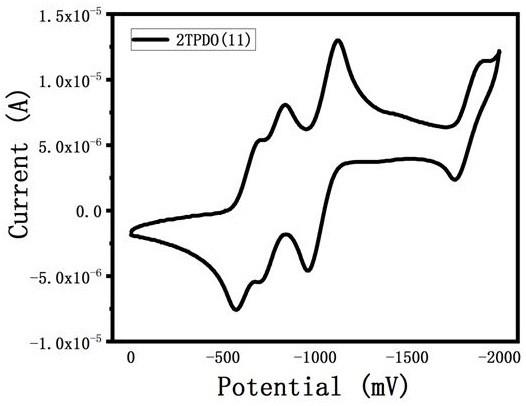
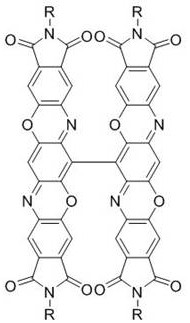
Triphenyl dioxazine imide diploid derivative and preparation method thereof
A technology of triphenyldioxazine imide and derivatives, which is applied in the field of diploid derivatives of triphenyldioxazine imide and its preparation, and achieves simple reaction conditions, good redox characteristics, and high electron-accepting ability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020]
[0021] Weigh 1 g of bromotriphenyldioxazinimide and 987.52 mg of Cu powder into a reaction flask, add 25 ml of dimethyl sulfoxide, and stir and react at 120° C. for 18 hours. After the reaction was complete, the reaction solution was spin-dried under reduced pressure, and 0.4 g of the product was separated by column chromatography, with a yield of 45%, HRMS: found 1127.1800.
Embodiment 2
[0023]
[0024] Weigh 1 g of bromotriphenyldioxazinimide and 782.76 mg of Cu powder into a reaction flask, add 25 ml of dimethyl sulfoxide, and stir and react at 110° C. for 15 hours. After the reaction was complete, the reaction solution was spin-dried under reduced pressure, and 0.5 g of product was obtained by column chromatography, with a yield of 55%, HRMS: found 1463.8280.
Embodiment 3
[0026]
[0027] Weigh 1 g of bromotriphenyldioxazinimide and 782.76 mg of Cu powder into a reaction flask, add 25 ml of nitrogen methyl pyrrolidone, and stir and reflux at 140° C. for 8 hours. After the reaction was complete, the reaction solution was spin-dried under reduced pressure, and 0.45 g of the product was obtained by column chromatography, with a yield of 49%, HRMS: found 1688.2600.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com