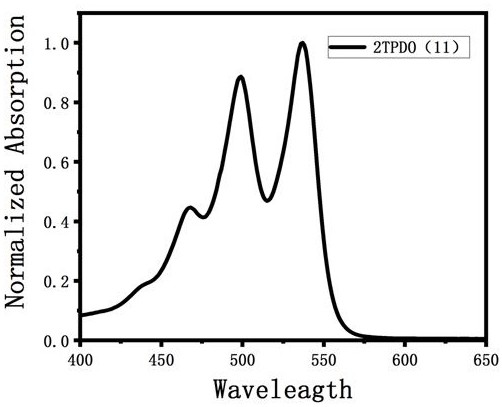
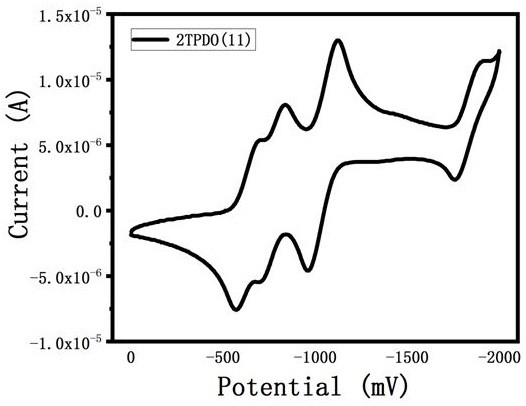
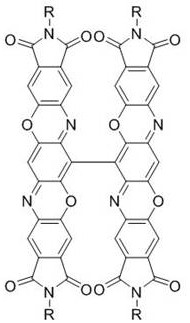
A class of triphenyl dioxazinimide diploid derivatives and preparation method thereof
A technology of triphenyldioxazinimide and its derivatives, applied in the field of diploid derivatives of triphenyldioxazinimide and its preparation, to achieve the effects of high molar extinction coefficient, low cost, and high electron-accepting ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020]
[0021] 1 g of bromotriphenyl dioxazine imide and 987.52 mg of Cu powder were weighed into a reaction flask, 25 ml of dimethyl sulfoxide was added, and the reaction was stirred at 120° C. for 18 hours. When the reaction was completed, the reaction solution was spin-dried under reduced pressure, and 0.4 g of product was obtained by column chromatography, with a yield of 45%, HRMS: found 1127.1800.
Embodiment 2
[0023]
[0024] 1 g of bromotriphenyl dioxazine imide and 782.76 mg of Cu powder were weighed into a reaction flask, 25 ml of dimethyl sulfoxide was added, and the reaction was stirred at 110° C. for 15 hours. When the reaction was complete, the reaction solution was spin-dried under reduced pressure, and 0.5 g of product was obtained by column chromatography, with a yield of 55%, HRMS: found 1463.8280.
Embodiment 3
[0026]
[0027] 1 g of bromotriphenyl dioxazine imide and 782.76 mg of Cu powder were weighed into a reaction flask, 25 ml of nitrogen methylpyrrolidone was added, and the mixture was stirred and refluxed at 140° C. for 8 hours. When the reaction was complete, the reaction solution was spin-dried under reduced pressure, and 0.45 g of product was obtained by column chromatography, with a yield of 49%, HRMS: found 1688.2600.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com