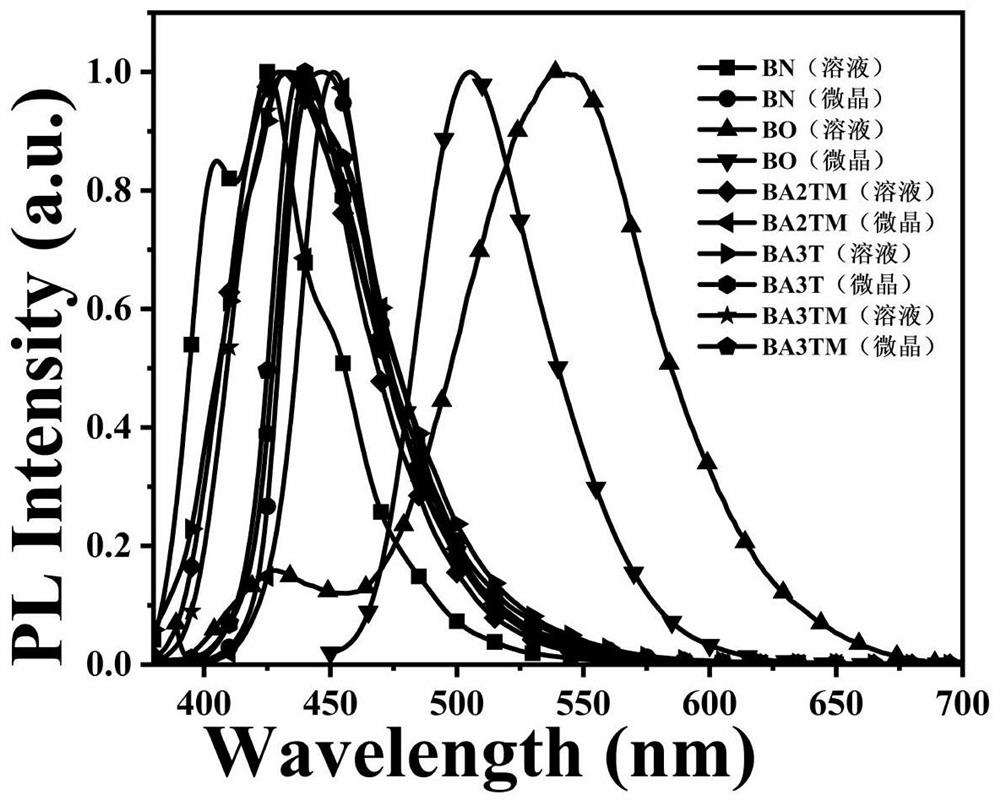
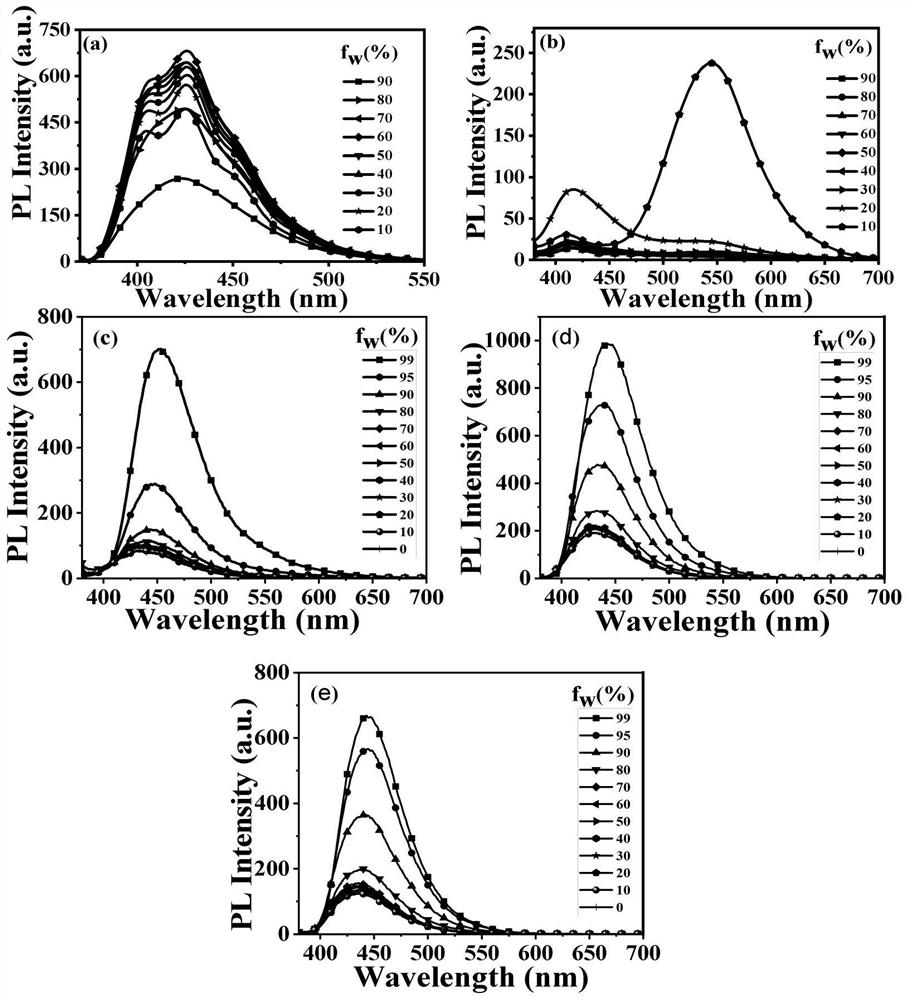
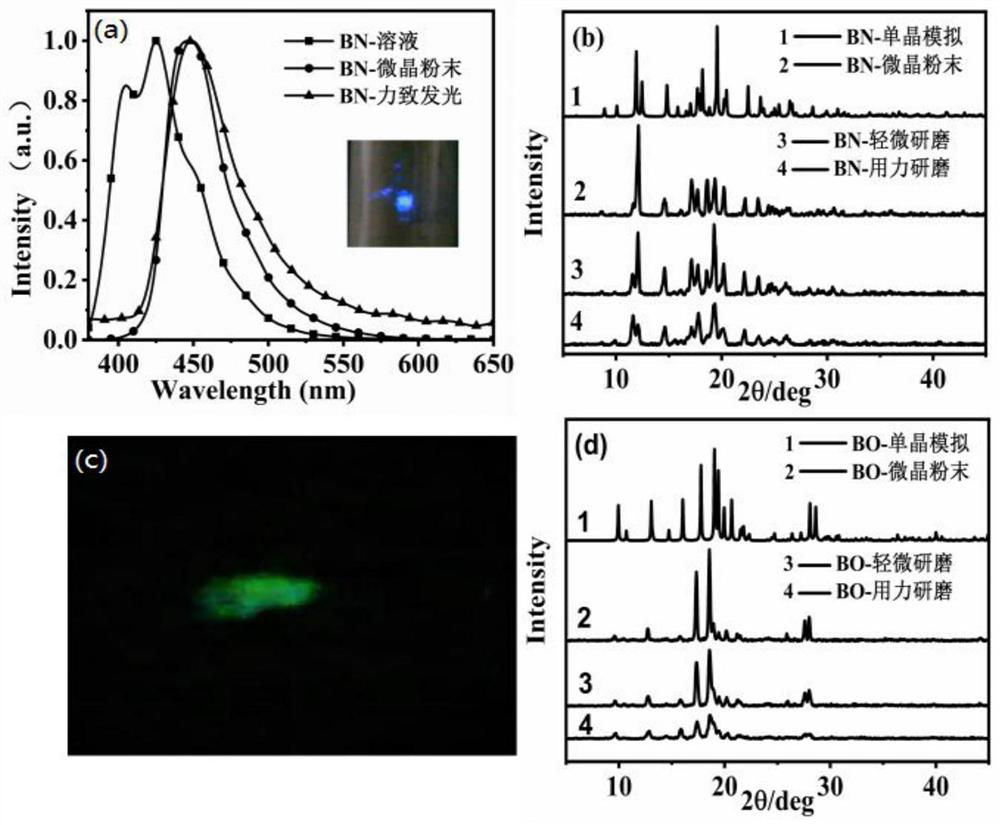
Anthracene-based mechanoluminescent organic material, and preparation method and application thereof
An organic material and luminescent technology, applied in luminescent materials, organic chemical methods, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] The present invention provides a method for preparing the mechanoluminescent organic material described in the above technical solution, comprising the following steps:
[0042] When R is hydrogen, mix 9-bromoanthracene, pinacol diborate, [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride, potassium acetate and the first organic solvent , carry out the Miyaura boronate reaction to obtain the mechanoluminescent organic material BN;
[0043] When R is p-nitrophenyl:
[0044] Miyaura Reaction, obtain 10-bromoanthracene boronate, denoted as compound 1;
[0045]Mix the compound 1, p-nitrophenylboronic acid, tetrakis(triphenylphosphine)palladium, potassium carbonate aqueous solution and a third organic solvent, and perform the first Suzuki reaction to obtain an anthracene-based mechanoluminescent organic material BO;
[0046] When R is thiophene or 2-methylthiophene, the compound 1, boronic acid-substituted thiophene compound, tetrakis(triphenylphosphine)palladium,...
Embodiment 1
[0078] Synthesis of compound 9-anthracene pinacol borate (BN):
[0079]
[0080] 9-Bromoanthracene (257.1 mg, 1 mmol), pinacol diboronate (380.9 mg, 1.5 mmol), [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride (24.5 mg, 0.03mmol) and potassium acetate (588.8mg, 6mmol) were dissolved in 10mL 1,4-dioxane, heated and stirred at 80°C under nitrogen for 24 hours, the cooled mixture was washed with water, and then two Chloromethane (DCM) extraction, anhydrous magnesium sulfate drying to remove solvent, and then using DCM / petroleum ether (1:4, v / v) as eluent, the resulting crude product was purified by silica gel column chromatography to obtain 9- Anthracene pinacol borate, denoted as BN, yield was 80.3%.
[0081] The NMR data of the compound BN prepared in this embodiment are: 1 H NMR (500MHz, DMSO), 8.67(s, 1H), 8.31(d, J=8.5Hz, 2H), 8.11(d, J=8.2Hz, 2H), 7.597.50(m, 4H), 1.52( s, 12H). MALDI-TOF-MS: 303.89.
Embodiment 2
[0083] Synthesis of 10-bromoanthracene borate (compound 1):
[0084]
[0085] 9,10-Dibromoanthracene (336.2mg, 1mmol), pinacol diborate (380.9mg, 1.5mmol), [1,1'-bis(diphenylphosphino)ferrocene] dichloride A mixture of palladium (24.5mg, 0.03mmol) and potassium acetate (588.8mg, 6mmol) was dissolved in 10mL of 1,4-dioxane gas, heated and stirred at 80°C for 24 hours, after cooling, the resulting mixture was washed with water, DCM Extract, then dry with anhydrous magnesium sulfate for 12h to remove the solvent, then use DCM / petroleum ether (1:4, v / v) as the eluent, and purify the obtained crude product by silica gel column chromatography to obtain compound 1. The rate is 85.3%, 325.5mg ( 1 H NMR (500MHz, DMSO), 8.50(t, J=8.2Hz, 2H), 8.29(t, J=8.2Hz, 2H), 7.827.61(m, 4H), 1.54(d, J=8.1Hz, 12H). MALDI-TOF-MS: 382.39);
[0086] Synthesis of 10-nitrophenyl anthracene boronate (BO):
[0087]
[0088] Add compound 1 (500mg, 1.31mmol), p-nitrophenylboronic acid (327mg, 1.96...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com