African swine fever virus CD2v extracellular domain recombinant protein and application thereof
A technology of African swine fever virus and recombinant protein, applied in the field of African swine fever virus CD2v ectodomain recombinant protein, ELISA detection kit for detection of African swine fever virus, can solve the problems of cumbersome operation, limited application, inconsistency, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
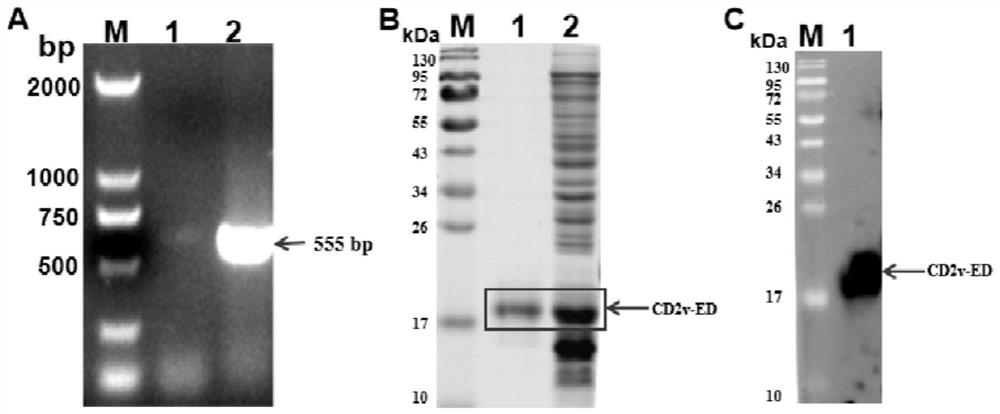
[0058] Example 1 Amplification of CD2v Extracellular Domain Gene and Construction of Recombinant Plasmid
[0059] Using the ASFV SY-18 genome CD2v gene published in GenBank as a template, the CD2v ectodomain gene was synthesized by Beijing Qingke Biotechnology Co., Ltd. as a PCR amplification template, and the CD2v ectodomain amplification primers were designed as follows to amplify the CD2v ectodomain Gene:
[0060] Upstream primers:
[0061] 5'-TAAGAAGGAGATATACCATGGATTATTGGGTTAGTTTTTAATAAACAATAATTTTAGATAG-3';
[0062] Downstream primers:
[0063] 5'-GTGGTGGTGGTGGTGCTCGAGTTAAGTATAAAAATAGTTAGATGACAATG-3'.
[0064] Platinum SuperFi II Green PCRmaster mix from Thermo Fisher Scientific was used for PCR amplification, and the amplification system included:
[0065] 2×Platinum SuperFi II Green buffer 12.5μL;
[0066] 5×GC buffer 5μL;
[0067] 1 μL each of upstream primer and downstream primer;
[0068] Template 2.5 μL;
[0069] wxya 2 O 3 μL;
[0070] The total volume of ...
Embodiment 2CD2
[0074] Example 2 Induced expression, purification and identification of CD2v extracellular domain protein
[0075] Induce the expression of CD2v ectodomain protein on the positive clones with correct sequencing, and wait for the bacterial solution OD 600nm Add isopropylthiogalactopyranoside (IPTG) with a final concentration of 500 μM between 0.6 and 0.8, induce 8 hours on a shaker at 37°C at 180 rpm, collect the bacteria, add sterile PBS to resuspend the bacteria, and perform ultrasonic disruption. Ultrasonic parameters are: power 30W; emission 5s, intermittent 5s. After the sonication, centrifuge at 12000rpm and 4°C for 20min, collect the supernatant and precipitate, add 5× protein loading buffer respectively for SDS-PAGE protein electrophoresis and western blot (Western Blot, WB) identification. The correctly identified protein bands were developed with 0.3M KCl for 5 minutes, and then the gel was cut. The recovered gel was purified by electrophoresis in a 3500D dialysis ba...
Embodiment 3
[0077] Example 3 Condition optimization of indirect ELISA coated with CD2v extracellular domain protein
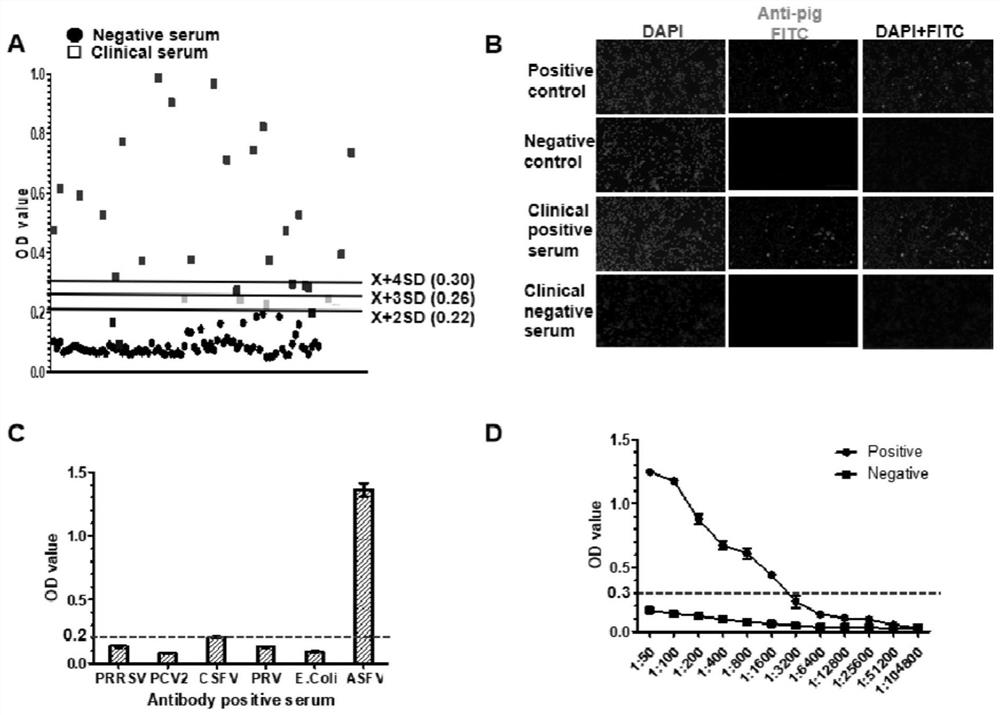
[0078] The CD2v ectodomain protein purified in Example 2 was coated with ELISA, and the optimal antigen coating concentration and the optimal clinical test sample were carried out in the mode of checkerboard method (in this embodiment, the antibody-positive African swine fever virus Determination of dilution of standard pig serum instead of clinical serum samples).
[0079] The antigen concentration gradients were controlled at 1 μg / mL, 2 μg / mL, 4 μg / mL, and 8 μg / mL; the dilution gradients of clinical serum samples were 1:50, 1:100, 1:200, and 1:400. Positive sample OD 450nm Value and negative sample OD 450nm The antigen coating concentration and clinical serum sample dilution when the value ratio is the largest and the positive sample OD value is close to 1 are the best antigen coating concentration and the best clinical serum sample dilution. The results are shown in T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com