Ambroxol hydrochloride injection and preparation method thereof
A technology of ambroxol hydrochloride and injection, which is used in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc., can solve problems such as poor stability, adverse reactions, slow dissolution speed, etc. High stability and safety, low adverse reaction rate, suitable heating temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
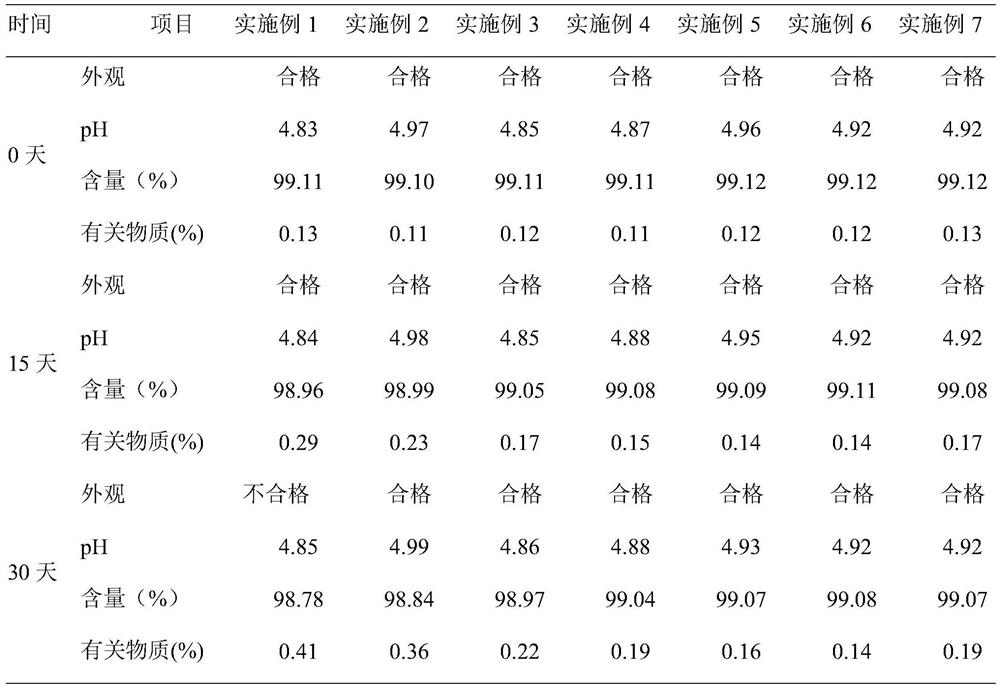
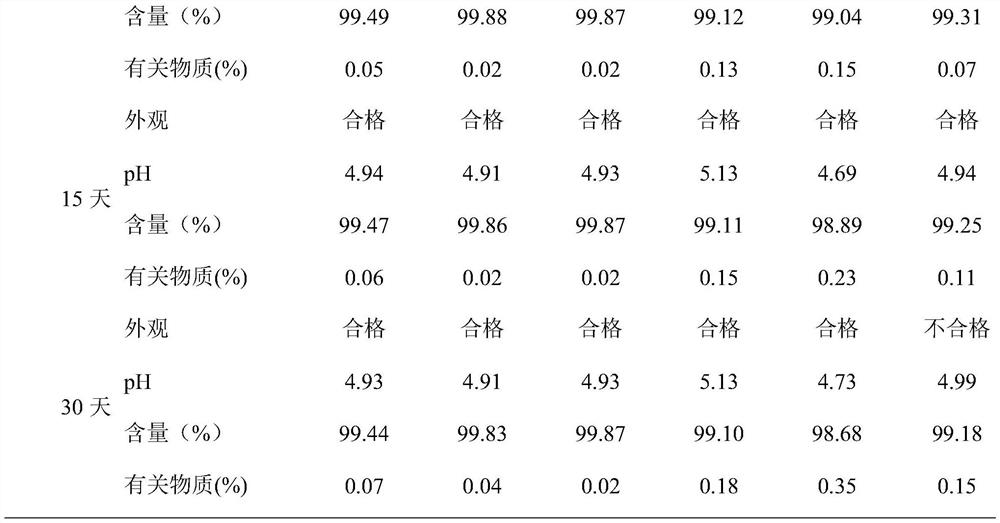
Examples
Embodiment 1
[0031] An ambroxol hydrochloride injection, which contains 75 mg of ambroxol hydrochloride, 14 mg of sodium citrate, 4 mg of citric acid, 0.8 mg of methionine, and water for injection is added to 10 ml.
[0032] The preparation method comprises: (1) taking 75% of the prescribed amount of water for injection, adding the prescribed amount of sodium citrate and citric acid, stirring at 150 rpm for 15 minutes to completely dissolve it, and controlling the pH to 4.8; (2) adding the prescribed amount of hydrochloric acid Ambroxol, stirred at 200rpm for 25min; (3) Add the prescribed amount of methionine and the remaining water for injection, stir for 30min, filter through a 0.22 μm microporous membrane, fill, and fill with nitrogen protection during the whole preparation and filling process, Sterilize at 121°C for 15 minutes.
Embodiment 2
[0034] An ambroxol hydrochloride injection, which contains 75 mg of ambroxol hydrochloride, 18 mg of disodium hydrogen phosphate, 12 mg of phosphoric acid, 90 mg of sodium chloride, 1.2 mg of cysteine, and water for injection is added to 10 ml.
[0035] The preparation method comprises: (1) taking 85% of the formula quantity of water for injection, adding disodium hydrogen phosphate and phosphoric acid in the prescription quantity, stirring at 200 rpm for 10 minutes to completely dissolve it, and controlling the pH to be 4.9; (2) adding the formula quantity of ammonium bromide hydrochloride (3) Add the prescribed amount of cysteine, sodium chloride and the remaining water for injection, stir for 35 minutes, filter through a 0.22 μm microporous membrane, fill, and fill with nitrogen during the entire preparation and filling process Protected and sterilized at 121°C for 15 minutes.
Embodiment 3
[0037] An ambroxol hydrochloride injection, containing 75 mg of ambroxol hydrochloride, 18 mg of disodium hydrogen phosphate, 8 mg of citric acid, 100 mg of glucose, 1.0 mg of L-proline, and 10 ml of water for injection.
[0038] The preparation method comprises: (1) taking 65% of the prescribed amount of water for injection, adding the prescribed amount of disodium hydrogen phosphate and citric acid, stirring at 180 rpm for 12 minutes to completely dissolve it, and controlling the pH to 4.9; (2) adding the prescribed amount of hydrochloric acid Ambroxol, stirred at 160rpm for 25min; (3) Add prescription amount of L-proline, glucose and remaining water for injection, stir for 25min, filter through 0.22 μm microporous membrane, fill, and fill in the whole preparation and filling process Nitrogen protection, sterilized at 121°C for 15 minutes, ready to use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com