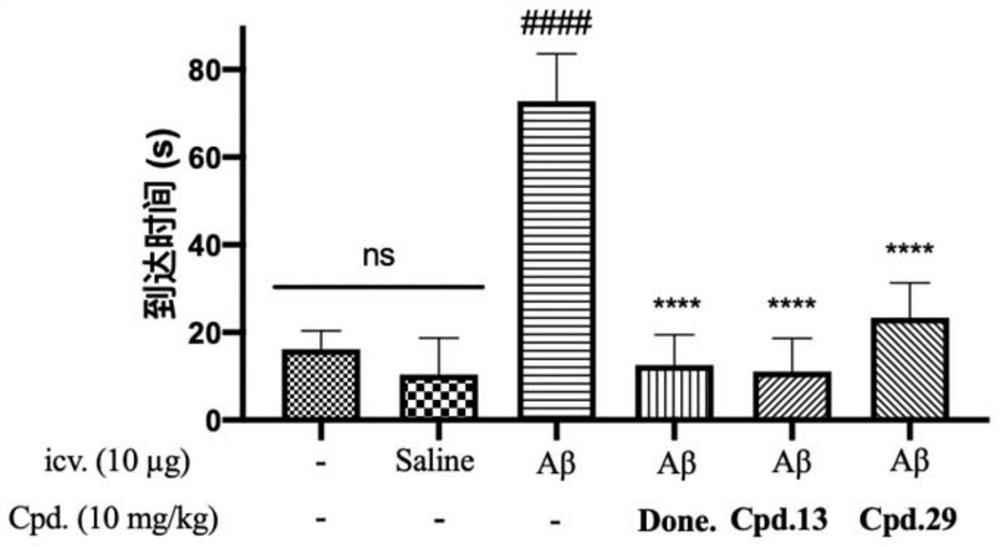
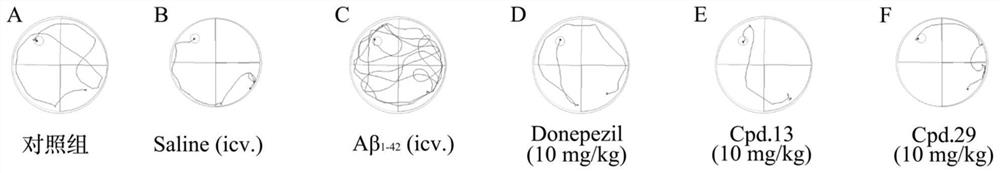
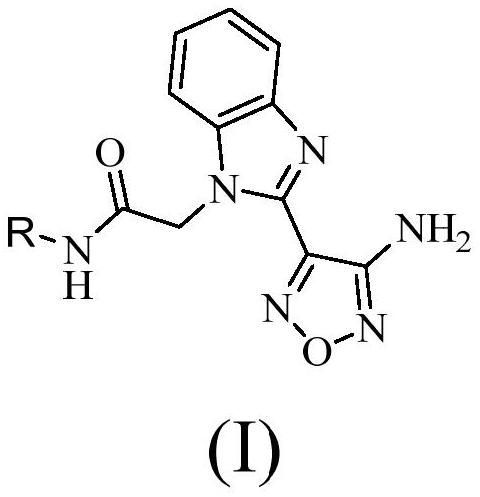
Compound with benzimidazole structure and preparation method and application thereof
A compound, benzo technology, applied in the field of medicine, can solve the problems of poor selectivity, lack of structural novelty and diversity, and small quantity, and achieve the effect of high activity in vivo
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Synthesis of 4-(1H-benzo[d]imidazol-2-yl)-1,2,5-oxadiazol-3-amine (Intermediate 1)
[0034]Take 2-cyanomethylbenzimidazole (1g, 6.36mmol) in an eggplant-shaped bottle, dissolve it with acetic acid (10ml), add an aqueous solution of sodium nitrite (0.44g, 6.36mmol) dropwise under an ice bath, and a solid is formed, After stirring in an ice bath for 40 minutes, it was suction filtered, and the filter cake was washed once with water and twice with diethyl ether. Take another eggplant-shaped bottle, add hydroxylamine hydrochloride (0.53g, 7.63mmol) in an ice bath, add water as a solvent, add potassium hydroxide (0.54g, 9.54mmol), and then add diglyme (6ml) , and finally add the filter cake of the previous step, return the reaction system to room temperature and start heating, reflux for 6 hours, and cool to room temperature; a large number of golden pink crystals precipitate, filter with suction, wash the filter cake once with water, wash twice with ether, and dry to ob...
Embodiment 2
[0042] 2-(2-(4-Amino-1,2,5-oxadiazol-3-yl)-1H-benzo[d]imidazol-1-yl)-N-(3-(pyrrolidin-1- Base) the synthesis of phenylacetamide:
[0043] With reference to the synthetic method of Example 1, the 3-aminobenzoic acid in Example 1 is replaced by 3-(pyrrolidin-1-yl)aniline to obtain a brownish black solid compound, which is 2-(2-(4-amino- 1,2,5-oxadiazol-3-yl)-1H-benzo[d]imidazol-1-yl)-N-(3-(pyrrolidin-1-yl)phenylacetamide (compound 2) .TLC detection is one point, there are dark spots under the ultraviolet lamp at 254nm, and there is no fluorescence at 365nm. 1 H NMR (300MHz, DMSO-d6): δ10.37(s,1H,N H CO), 7.96-7.70 (m, 2H, ArH), 7.63-7.32 (m, 2H, ArH), 7.09 (t, J=8.1Hz, 1H, ArH), 7.04 (s, 2H, N H 2 ), 6.89(t, J=2.1Hz, 1H, ArH), 6.80(dd, J=7.7, 1.8Hz, 1H, ArH), 6.28(dd, J=8.2, 2.3Hz, 1H, ArH), 5.58( s,2H,C H 2 ),3.26-3.06(m,4H,C H 2 C H 2 ),2.15-1.82(m,4H,C H 2 C H 2 ).
Embodiment 3
[0045] 2-(2-(4-Amino-1,2,5-oxadiazol-3-yl)-1H-benzo[d]imidazol-1-yl)-N-(3-(piperidine-1- Base) phenyl) acetamide synthesis:
[0046] With reference to the synthetic method of Example 1, the 3-aminobenzoic acid in Example 1 is replaced by 3-(piperidin-1-yl)aniline to obtain a brown solid compound, which is 2-(2-(4-amino- 1,2,5-oxadiazol-3-yl)-1H-benzo[d]imidazol-1-yl)-N-(3-(piperidin-1-yl)phenyl)acetamide (compound 3 ). TLC detection is one point, there are dark spots under the ultraviolet lamp at 254nm, and there is no fluorescence at 365nm. 1 H NMR (300MHz, DMSO-d6): δ10.85(s,1H,N H CO),7.99(s,1H,ArH),7.88(dd,J=15.3,7.9Hz,2H,ArH),7.47(d,J=7.6Hz,2H,ArH),7.42(t,J=7.5Hz ,1H,ArH),7.30(d,J=24.6Hz,2H,ArH),7.05(s,2H,N H 2 ),5.65(s,2H,C H 2 ),3.47-3.38(m,4H,C H 2 C H 2 ), 1.81(t, J=5.8Hz, 4H, C H 2 C H 2 ),1.63(s,2H,C H 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com