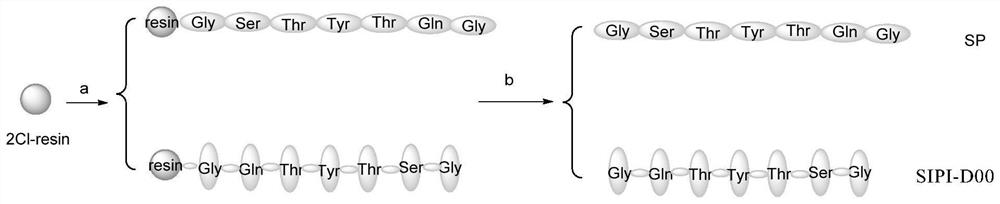
Biological peptide for treating asthma and application of biological peptide
An asthma and drug technology, applied in the field of biological peptides for the treatment of asthma, can solve the problems of difficult asthma, poor stability of peptides, and effective treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
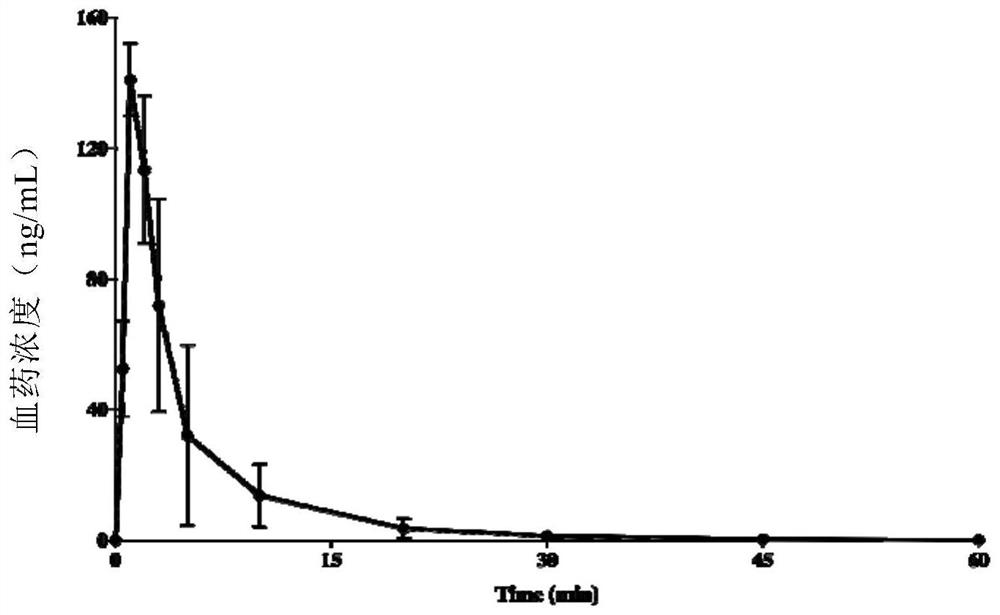
[0203] Each 7-peptide compound stability in the rat whole blood of embodiment 1
[0204] 1.1 Experimental procedure and incubation conditions
[0205] Preparation of test samples: Dissolve and dilute SP and SIPI-D00 with an appropriate amount of 50% acetonitrile water containing 0.1% formic acid to obtain a working solution with a concentration of 200 μg / mL. Table 1 below shows the usage conditions of the stability study reagents.
[0206] Table 1 Stability study reagent usage
[0207]
[0208] 1) Add fresh rat whole blood to the EP tube, shake gently to mix;
[0209] 2) EP tubes were pre-incubated in a 37°C water bath for 5 minutes;
[0210] 3) Add each compound to initiate the reaction, and continue to incubate at 37°C;
[0211] 4) At 0, 2 min, 5 min, 10 min, 30 min, and 60 min, take 50 μL from the sample tube to the stop tube, add 50 μL of ice-cold 7% perchloric acid, oscillate, and terminate the reaction;
[0212] 5) Centrifuge at 13000 r / min for 10 min, absorb 75 ...
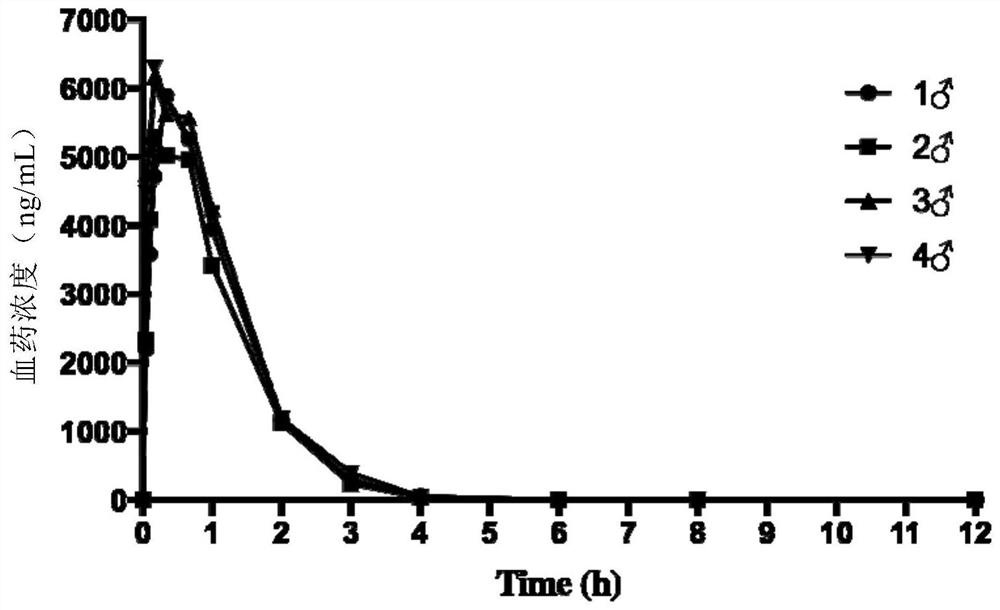
Embodiment 2
[0218] Example 2 Plasma drug concentration at different times after a single subcutaneous injection of SP peptide or SIPI-D00 peptide in SD rats
[0219] 1. Materials
[0220] SP peptide (amino acid sequence SEQ ID NO: 1)
[0221] SIPI-D00 polypeptide (amino acid sequence SEQ ID NO: 2)
[0222] 2. Experimental animals
[0223] Male SD rats with a body weight of about 200g were reared in an air-conditioned constant temperature room at room temperature of 20-24°C, humidity of 40-70%, light for 12 hours, and free access to food and water.
[0224] 3. Experimental method
[0225] 3.1 Administration method
[0226] Route: single dorsal subcutaneous injection;
[0227] Dosage of drugs: SP peptide and SIPI-D00 peptide were prepared into 3.33 mg / mL solution with normal saline, the dosage of SP peptide subcutaneously injected on the back was 10 mg / kg body weight, and the administration of SIPI-D00 peptide subcutaneously injected on the back The dose is 5mg / kg body weight.
[022...
Embodiment 3
[0261] Example 3 Effect of SP Peptide and SIPI-D00 Peptide on ConA (Concanavalin A) Stimulation of Mouse Splenocytes to Secrete IL-4
[0262] Asthma is mainly an inflammatory disease mediated by Th2 cells. During disease progression, Th2 cells secrete IL-4, IL-5, and IL-13, and these cytokines act on eosinophils, mast cells, and airway structural cells to promote the accumulation of inflammatory cells in the bronchial mucosal epithelium and play a refactoring. Therefore, by examining the effects of SP peptide and SIPI-D00 peptide on the secretion of IL-4 to evaluate their therapeutic effect on asthma.
[0263] 1. Experimental method
[0264] Three BALB / C mice were sacrificed by cervical dislocation after anesthesia. Soak in 75% ethanol for 5 minutes. Dissect, take spleen, prepare splenic lymphocytes, after counting, add 2×10 5 One per well, different concentrations of SP peptide and SIPI-D00 peptide were set up, and different concentrations of CsA (cyclosporine A) were used...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com