Method for detecting content of impurity C in gliquidone
A technology for gliquidone and impurities, applied in the field of drug analysis, can solve the problems of ineffective detection of impurity C, serious adverse reactions, and toxicity, and achieve the effects of ensuring drug safety, clear principles, and improving quality standards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 1. Instruments and testing conditions
[0037] The Agilent 1260 high-performance liquid chromatograph produced by U.S. Agilent Corporation, the AgilentZORBAX Eclipse Plus C18 chromatographic column produced by Agilent; Detection wavelength is 210nm; Mobile phase: methyl alcohol: phosphate aqueous solution (phosphate aqueous solution pH value is 4.5), in 20 minutes The internal temperature was changed from 45:55 to 70:30; the flow rate was 1ml / min; the column temperature of the chromatographic column was 35°C; the injection volume was 20μl.
[0038] 2. Experimental steps
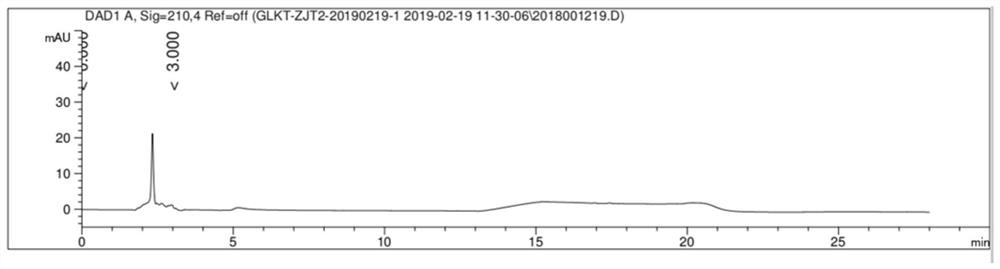
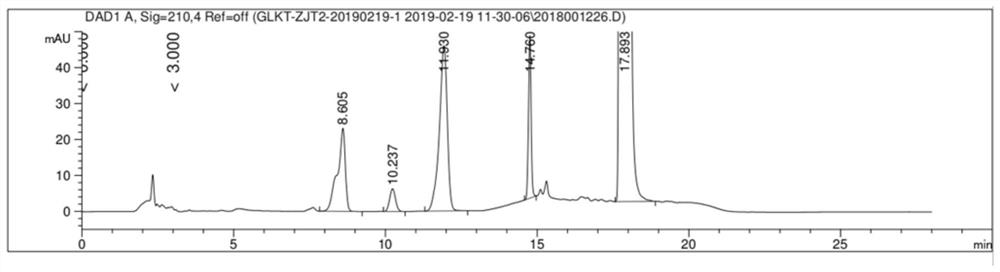
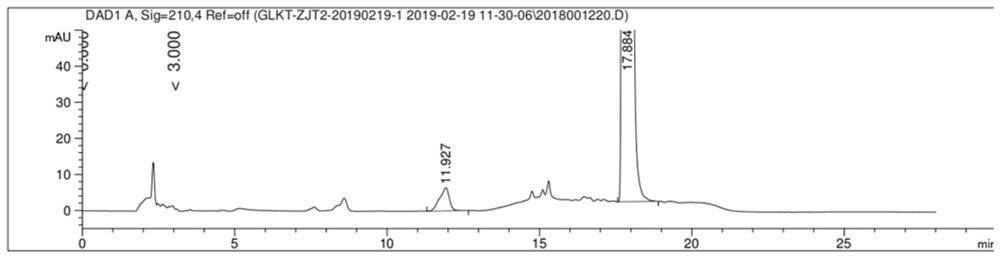
[0039] Take an appropriate amount of the mixture composed of gliquidone, impurity C, impurity A, impurity G, impurity J and impurity L, add methanol to dissolve, and prepare a mixed solution containing 5mg gliquidone and 0.05mg of each impurity per 1ml; Liquinone and the standard substances of various impurities were prepared into positioning solution as a control. Get the blank solution, the mixed s...
Embodiment 2
[0041] 1. Instruments and testing conditions
[0042] The Agilent 1260 high-performance liquid chromatograph produced by U.S. Agilent Company, the AgilentZORBAX Eclipse Plus C18 chromatographic column produced by Agilent; The detection wavelength is 210nm; Mobile phase: acetonitrile: aqueous acetate solution (the pH value of aqueous acetate solution is 4.5), in Change from 45:55 to 70:30 within 18 minutes; the flow rate is 1ml / min; the column temperature of the chromatographic column is 35°C; the injection volume is 20μl.
[0043] 2. Experimental steps
[0044] Take an appropriate amount of impurity C, add methanol to dissolve, and prepare a solution containing 1 μg of impurity C per 1 ml, as the limit of quantitation solution, prepare 2 copies in parallel; take an appropriate amount of impurity C, add methanol to dissolve, and prepare a solution containing 0.3 μg of impurity C per 1 ml , as the limit of detection solution, prepare 2 parts in parallel. Sampling was carried o...
Embodiment 3
[0048] 1. Instruments and testing conditions
[0049] The Agilent 1260 high-performance liquid chromatograph produced by U.S. Agilent Corporation, the AgilentZORBAX Eclipse Plus C18 chromatographic column produced by Agilent; Detection wavelength is 210nm; Mobile phase: methyl alcohol: phosphate aqueous solution (phosphate aqueous solution pH value is 4.5), in 20 minutes The internal temperature was changed from 40:60 to 65:35; the flow rate was 1ml / min; the column temperature of the chromatographic column was 35°C; the injection volume was 20μl.
[0050] 2. Experimental steps
[0051] Take an appropriate amount of impurity C, add methanol to dissolve, prepare a solution containing 40 μg of impurity C per 1 ml, and dilute it to a linear solution of different concentrations, and then inject samples for determination. The results showed that the impurity C had a good linear relationship within the concentration range of 1-40 μg / ml. The experimental results are shown in Table 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com