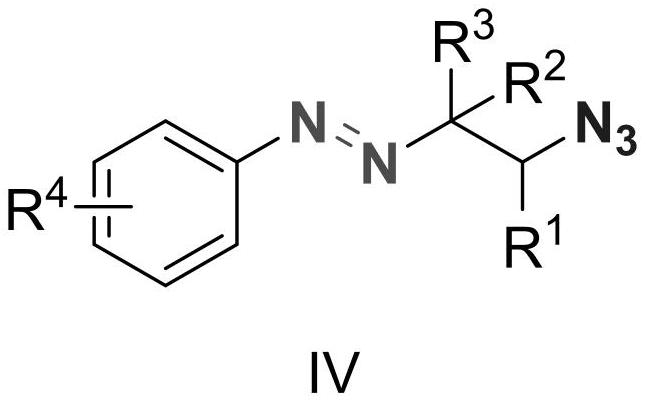
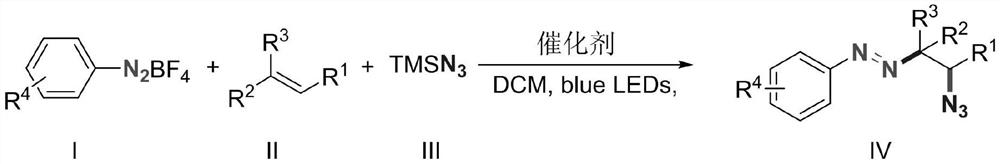
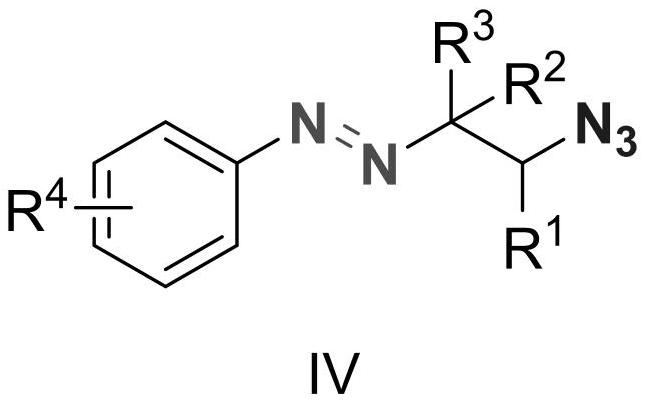
Alkyl aryl asymmetric azo and synthesis method thereof
A technique for the synthesis of alkyl aryl groups, which is applied in the field of alkyl aryl asymmetric azo and its synthesis, can solve the problems of increasing cytotoxicity and increasing the concentration of cisplatin in cells, and achieves good research and application value Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018]
[0019] Diazonium p-methoxybenzenetetrafluoroborate (2.20g, 10mmol), 1-pentene (1.40g, 20mmol), azidotrimethylsilane (2.30g, 20mmol) and 9-trimethylphenyl- 10-Methylacridine perchlorate (411mg, 0.1mmol) was added to the reaction flask, dichloromethane (50g) was added to dissolve it, and a 10W blue LED light was used as the light source for illumination, and the reaction was stirred at room temperature for 15 minutes. After the reaction was completed, a saturated aqueous sodium bicarbonate solution (50 g) was added to quench the reaction, dichloromethane (50 g) was added, and after sufficient stirring, the mixture was allowed to stand for layering. The organic layer was dried over anhydrous magnesium sulfate, filtered, and concentrated to obtain a brown crude product. The crude product was purified by column method (eluent: ethyl acetate / petroleum ether=1:50, v / v) to obtain 2.35 g of the target product as a dark yellow liquid with a yield of 95%. 1 HNMR (500MHz, CDCl...
Embodiment 2
[0021]
[0022] Diazonium p-methoxybenzenetetrafluoroborate (2.20g, 10mmol), 1-hexene (1.68g, 20mmol), azidotrimethylsilane (2.30g, 20mmol) and 9-trimethylphenyl- 10-Methylacridine perchlorate (411mg, 0.1mmol) was added to the reaction flask, dichloromethane (50g) was added to dissolve it, and a 10W blue LED light was used as the light source for illumination, and the reaction was stirred at room temperature for 15 minutes. After the reaction was completed, a saturated aqueous sodium bicarbonate solution (50 g) was added to quench the reaction, dichloromethane (50 g) was added, and after sufficient stirring, the mixture was allowed to stand for layering. The organic layer was dried over anhydrous magnesium sulfate, filtered, and concentrated to obtain a brown crude product. The crude product was purified by column method (eluent: ethyl acetate / petroleum ether=1:50, v / v) to obtain 2.4 g of the target product as dark yellow liquid with a yield of 92%. 1 HNMR (500MHz, CDCl 3 ...
Embodiment 3
[0024]
[0025] Diazonium p-methoxybenzenetetrafluoroborate (2.20g, 10mmol), 1-octene (2.24g, 20mmol), azidotrimethylsilane (2.30g, 20mmol) and 9-mesityl- 10-Methylacridine perchlorate (411mg, 0.1mmol) was added to the reaction flask, dichloromethane (50g) was added to dissolve it, and a 10W blue LED light was used as the light source for illumination, and the reaction was stirred at room temperature for 15 minutes. After the reaction was completed, a saturated aqueous sodium bicarbonate solution (50 g) was added to quench the reaction, dichloromethane (50 g) was added, and after stirring, the mixture was allowed to stand for layering. The organic layer was dried over anhydrous magnesium sulfate, filtered, and concentrated to obtain a brown crude product. The crude product was purified by column method (eluent: ethyl acetate / petroleum ether=1:50, v / v) to obtain 2.75 g of dark yellow liquid with a yield of 95%. 1 H NMR (500MHz, CDCl 3 )δ7.64(d, J=8.5Hz, 2H), 6.88(d, J=8.6Hz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com