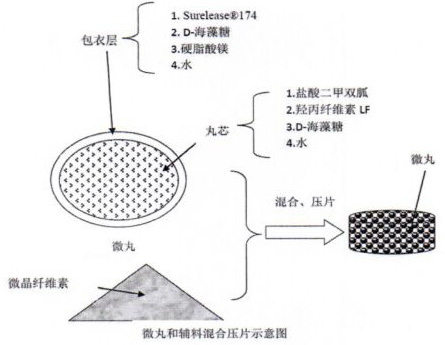
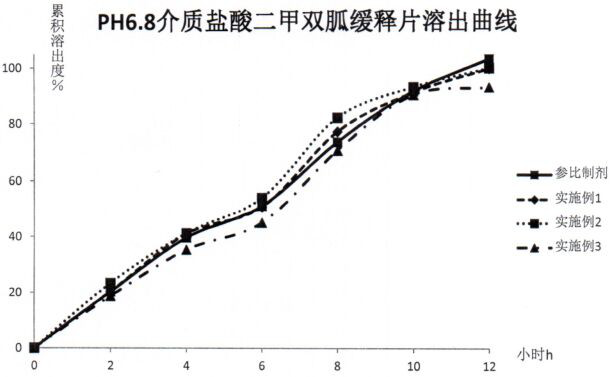
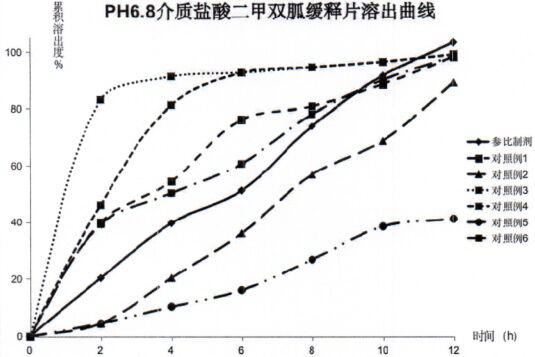
Metformin hydrochloride sustained release tablet composition
A technology of metformin hydrochloride and sustained-release tablets, which is applied in the directions of drug combinations, medical preparations of inactive ingredients, coatings, etc. Problems such as poor physical properties of the coating film, to achieve the effect of overcoming easy rupture
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0035] Experimental example 1. Prescription:
[0036] .
[0037] Prepare 1000 tablets according to the preparation method described in the prescription dosage and the technical scheme of the instructions.
Embodiment 2
[0038] Embodiment 2. Prescription:
[0039] .
[0040] Prepare 1000 tablets according to the preparation method described in the dosage of the prescription and the technical scheme of the instruction manual.
Embodiment 3
[0041] Embodiment 3. Prescription:
[0042] .
[0043] Prepare 1000 tablets according to the preparation method described in the prescription dosage and the technical scheme of the instructions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com