Expression vector of anti-SARS-COV-2 neutralizing antibody
A SARS-COV-2 and expression vector technology, which is applied in the direction of antibodies, vectors, nucleic acid vectors, etc., can solve the problems of convalescent plasma treatment limitations and inability to mass produce
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0279] Example 1: Anti-SARS-CoV-2 neutralizing antibodies
[0280] Materials and methods
[0281] human sample
[0282] Peripheral blood mononuclear cells (PBMC) from healthy control donors were collected from the Center Hospitalier Universitaire of Université Laval (Centre Hospitalier Universitaire of Université Laval; CHU-Université Laval), while those from COVID-19 survivors PBMCs were obtained from SunnyBrook Hospital, Toronto. Ethical approval from ethics committees was obtained from both institutions prior to sample collection, and all participants signed individual informed consent.
[0283] fluorescent cell sorting
[0284] SARS-CoV-2 virus-like particles ( VLP) (Medicago, Quebec, Canada) was biotinylated.
[0285] PBMCs from COVID-19 survivors were thawed and allowed to stand for 30 min before staining with 1 μg of biotinylated SARS-CoV-2 VLPs. Samples were then stained with viability dye (fixable viability dye eFluor, Thermo Fisher), A488-conjugated strepta...
example 2
[0294] Example 2: Characterization of Antibodies
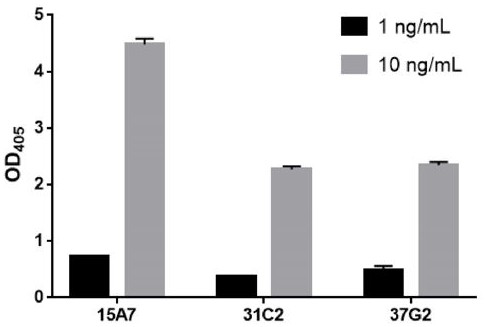
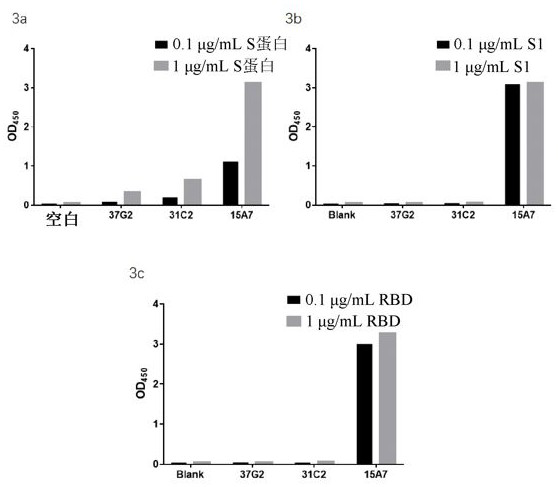
[0295] After screening all obtained antibodies by a SARS-CoV-2 specific binding assay (data not shown), the three best antibodies (i.e., 37G2, 31C2 and 15A7) were obtained, which were among the highest among the numerous antibodies we screened. showed the best binding affinity. figure 1 The SARS-CoV-2 specific binding activities of the three antibodies are shown in . The CDR regions of the antibodies were sequenced and listed in Table 1. As far as the applicant knows, the SARS-CoV-2 antibody containing the CDRs listed in Table 1 has not been reported before. Therefore, this application provides a new SARS-CoV-2 antibody to fight against the SARS-CoV-2 epidemic. Provides more options.
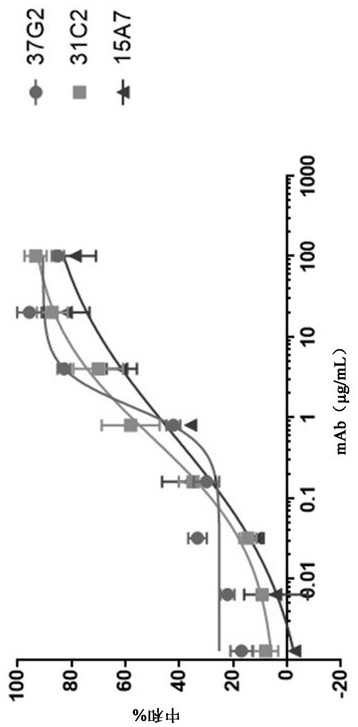
[0296] These 3 antibodies were recombinantly expressed and in vitro neutralization assays were performed in Vero-E6 cells using live virus. Such as figure 2 As shown in , all antibodies exhibited significant neutralizing capacity against S...
example 3
[0298] Example 3: AAV-mediated antibody gene delivery
[0299] The experiment was designed to test the effect of AAV-mediated antibody gene transfer in preventing SARS-CoV-2 viral challenge in mice.
[0300] AAV-mAb vector construction
[0301] Preparation of antibody expression cassette: Monoclonal antibody expression cassette was synthesized by Genscript and contained signal peptide, variable heavy chain domain, human IgG1 constant domain, followed by 2A self-cleavage peptide sequence, signal peptide, variable light chain domain and the human lambda constant domain. Antibody heavy chain and light chain nucleic acid sequences were codon optimized by GenScript. Each antibody expression cassette was amplified by PCR using Taq polymerase, and the PCR products were gel purified, and the band of interest was excised and purified using a gel extraction kit.
[0302] AAV vector preparation: The pAVA-00200 plasmid backbone contains the CASI promoter (Alejandro B. Balazs et al., ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com