Pharmaceutical composition, compound preparation and preparation method and application thereof
A technology of compound preparations and compositions, applied in the field of medicine, can solve the problems of large side effects, unsatisfactory therapeutic effects, slow onset of effects, etc., achieve convenience in taking medicine, inhibit 5-HT and/or NE reuptake, and relieve depression Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
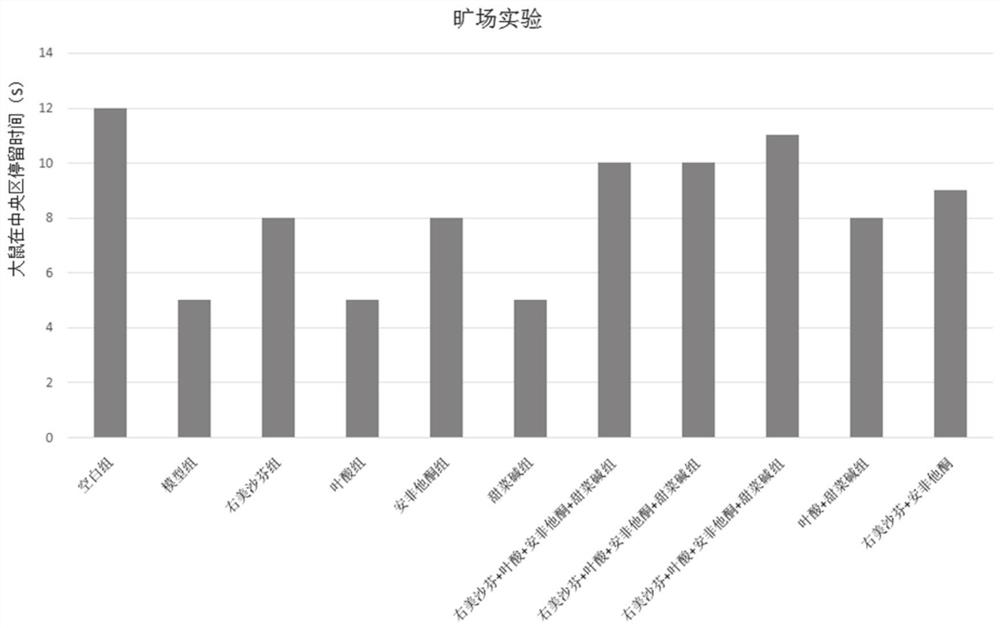
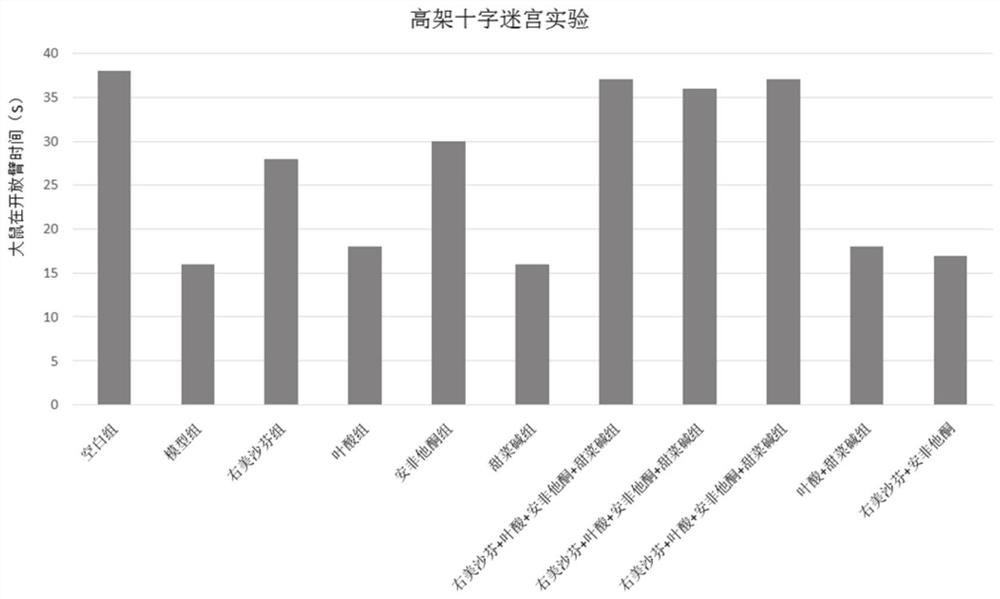
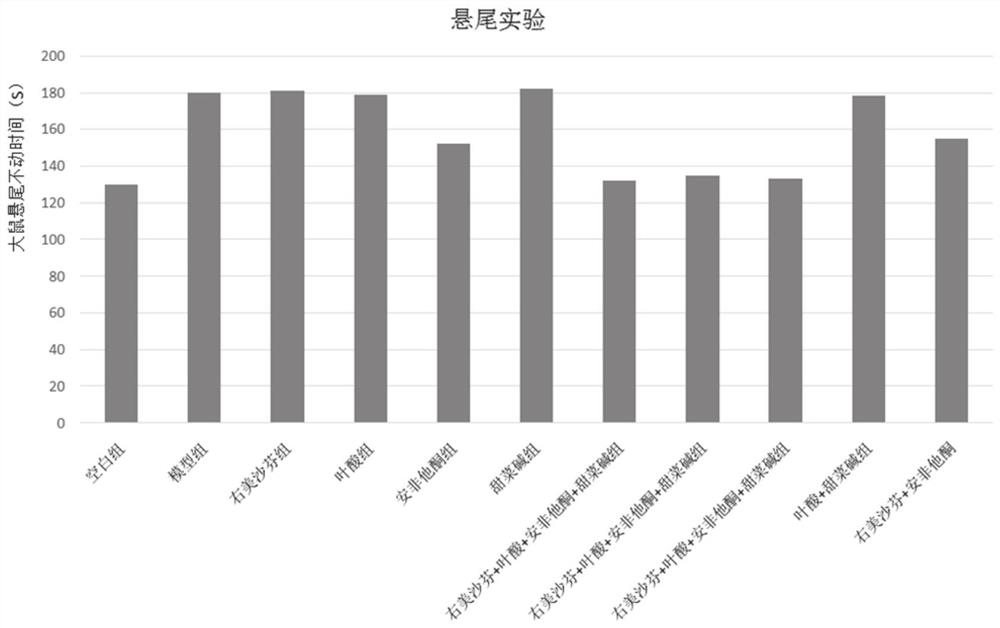
Image
Examples
Embodiment 1
[0140] The preparation of embodiment 1 compound tablet
[0141] The prescription is as follows (1000 tablets):
[0142]
[0143] Preparation method: crush the raw and auxiliary materials through an 80-mesh sieve, and set aside. Take by weighing dextromethorphan 15g, bupropion 30g, betaine 300g, carboxymethyl starch sodium 20g, calcium hydrogen phosphate 180g and evenly mix to form a mixture, and folic acid is dissolved in 200ml of 10% povidone aqueous solution (folic acid can dissolve completely That is, the amount of 10% povidone aqueous solution should not be too much), mix with the above-mentioned mixed material, then add the remaining 10% povidone aqueous solution, make soft material, 30 mesh granulation, add 5g magnesium stearate, mix , Determination of content, tableting, that is. During the preparation procedure, pay attention to avoid light. After the finished product passes the inspection, it will be packaged in aluminum and plastic. Keep away from light. Each...
Embodiment 12
[0151] Embodiment 12 prepares compound capsule
[0152] The prescription is as follows (1000 capsules):
[0153]
[0154] Preparation method: crush the raw and auxiliary materials through an 80-mesh sieve, and set aside. Take by weighing 50g of dextromethorphan, 100g of bupropion, 300g of betaine, 150g of lactose, 20g of sodium starch glycolate and evenly mix to form a mixture, and dissolve folic acid in 200ml of 10% povidone aqueous solution (folic acid can be completely dissolved). , the amount of 10% povidone aqueous solution should not be too much), mixed with the above-mentioned mixed materials, and then added the remaining 10% povidone aqueous solution to make soft materials, granulated with 30 mesh, dried at 60 ° C for 3 hours; granulated with 20 mesh, moisture Control it within 2.0%, add magnesium stearate in the prescribed amount, mix well, measure the content, pack into capsules, and get final product. During the preparation procedure, pay attention to avoid lig...
Embodiment 13~22
[0155] Embodiment 13~22 prepares compound capsule
[0156] The preparation process is the same as in Example 12. The composition and content of the 10 kinds of compound capsules are shown in Table 1, and the types and dosages of the 10 kinds of compound capsules are shown in Table 3.
[0157] Table 3 Types and dosages of excipients of 10 kinds of compound capsules.
[0158]
[0159]
[0160] Note: Adjust the dosage of 10% povidone according to the temperature and humidity, so that the soft material is suitable for the granulator.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com