Method for detecting beta-lactam antibiotics in enterovirus 71 inactivated vaccine
A technology of lactams and enteroviruses, which is applied in the field of antibiotic residue detection in vaccines, can solve the problems of unreported detection methods, and achieve the effects of good reproducibility and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1. Chemicals and reagents
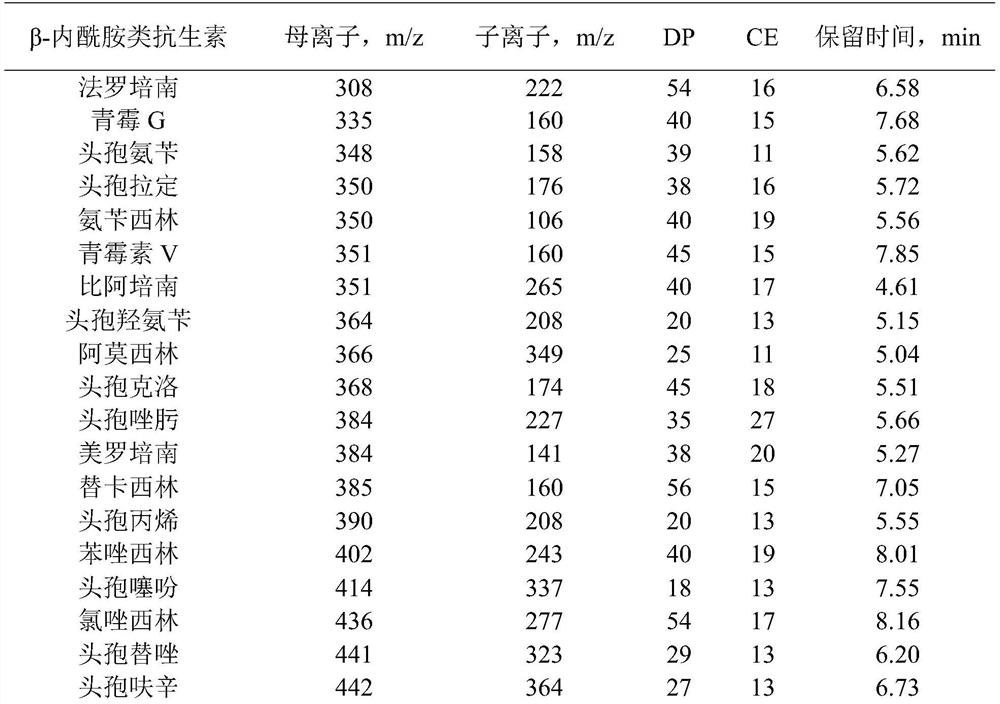
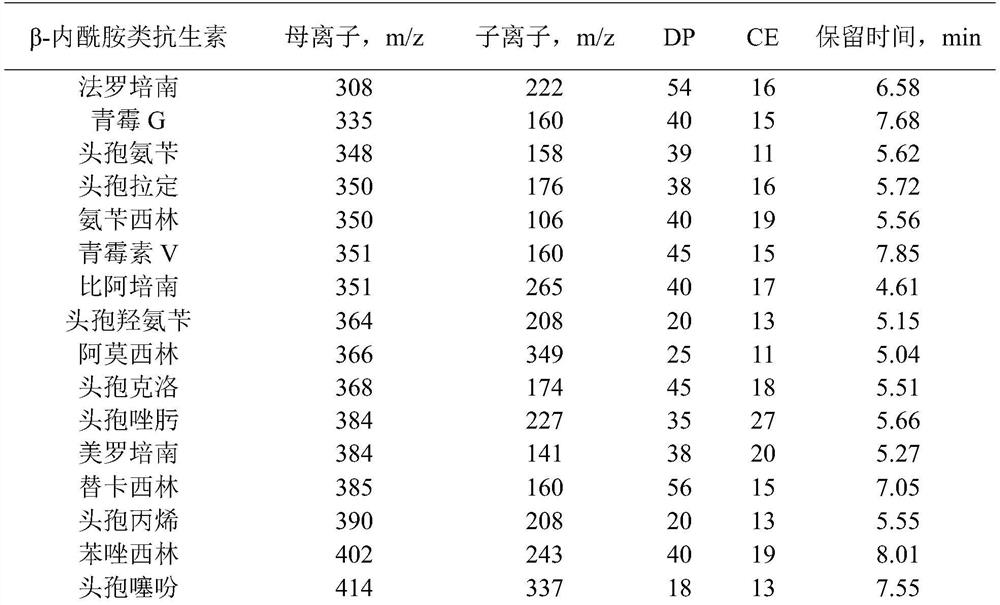
[0035]Reference substances faropenem (batch number 130532-201301, purity 80.3%), cefadroxil (batch number 130431-201203, purity 94.8%), oxacillin (batch number 130482-201402, purity 90.4%), cefixime (batch number 130503- 201706, purity 89.2%), cefepime (batch number 130524-201404, purity 83.5%), latamoxef (batch number 130590-201702, purity 84.3%), cefodizime (batch number 130520-201803, purity 89.8%), penicillin (batch number 130437-201707, purity 94.1%), amoxicillin (batch number 130409-201913, purity 86.9%), cephalothin (batch number 130407-200707, purity 96.1%), flucloxacillin (batch number 130529-202002, purity 92.6%) ), cefmenoxime (batch number 130525-201001, purity 94.6%), cefotiam (batch number 130565-201703, purity 80.7%), cefpiramide (batch number 130505-201301, purity 92.5%), cephalexin (batch number 130408- 201411, purity 94.4%), cefaclor (batch number 130481-201606, purity 95.3%), cefazolin (batch number 130421-201204, purity 9...
Embodiment 2
[0057] Optimization and efficiency of embodiment 2 extraction conditions
[0058] In the pre-treatment of the vaccine, the method of concentrating the vaccine in an ultrafiltration centrifuge tube and recovering the filtrate was first tried, but the matrix effect of this method was too large, and the purpose of extracting the target antibiotic was not achieved. We adopted a simple and rapid pretreatment solvent precipitation method, and used live attenuated varicella vaccine as a matrix to explore the effects of ethanol, methanol, and acetonitrile as precipitants. When the volume ratio of precipitant to vaccine was 1:1, the three kinds Precipitants, all have high matrix effects, and obvious matrix interference peaks can be seen, try to increase the amount of precipitants added, reduce matrix effects, and finally determine that precipitants should be added to live attenuated varicella vaccine (0.5mL / dose) The volume is 3 times of its specification, and the precipitation phenome...
Embodiment 3
[0059] The optimization of embodiment 3 liquid quality conditions
[0060] In this example, try to use the following three chromatographic columns: ACE Excel 5 C18 (75mm×2.1mm, 5μm), Shiseido CAPCSLL PAK C18 MG (150mm×2.0mm, 5μm), Shiseido CAPCSLL PAK C18 MG (75mm×2.0mm, 5 μm), the chromatographic conditions were optimized. The results showed that when the ACE Excel 5 C18 (75mm×2.1mm, 5μm) column was used, the amoxicillin peak split and the tailing was serious; when the Shiseido CAPCSLL PAK C18 MG (150mm×2.0mm, 5μm) was used as the The peak shape is sharp and the resolution is good; in order to shorten the retention time of each antibiotic, a Shiseido CAPCSLL PAK C18 MG (75mm×2.0mm, 5μm) chromatographic column was also used, but the peak shape of each antibiotic under this chromatographic column is relatively broad; so the final Choose Shiseido CAPCSLL PAK C18 MG (150mm×2.0mm, 5μm) chromatographic column.
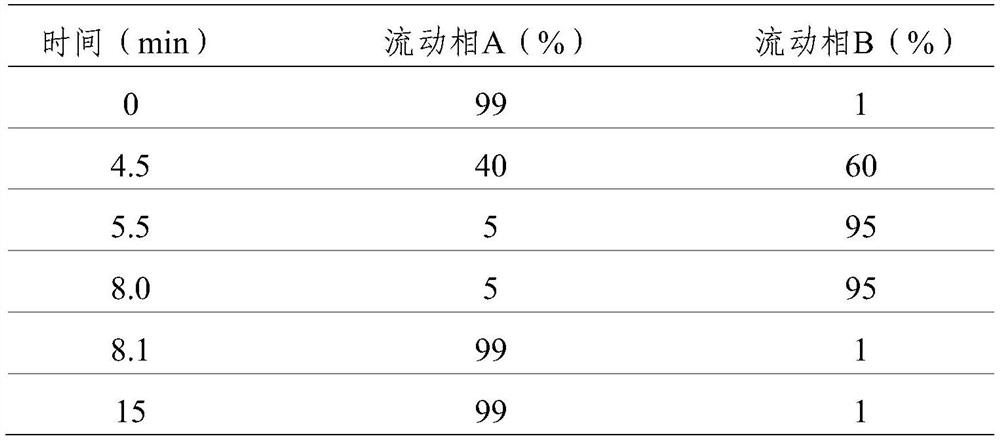
[0061]For the choice of mobile phase, mobile phase A: 5mM ammonium f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com