Method for high-value resource utilization of isobutene with low polymerization degree
A low-polymerization technology of isobutylene, which is applied in the field of converting low-polymerization isobutylene into multi-carbon mercaptans, achieves the effects of wide sources, environmental protection prices, and few by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
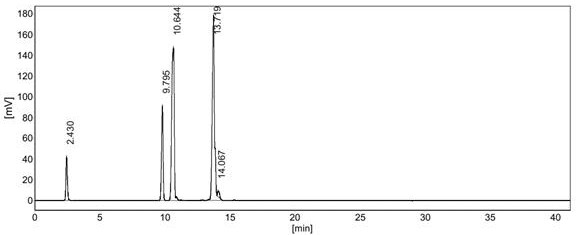
[0029] In a 316 stainless steel reactor with an inner diameter of 16mm and a length of 354mm, 30mL of dry Amberlyst 35 resin was loaded, and the catalyst was treated in situ under nitrogen, room pressure, and 110°C overnight; hydrogen sulfide was passed through a mass flow meter at 60mL / min Controlled feed into the reactor, triisobutene is passed into the reactor through a high-efficiency liquid phase pump at 3mL / h, hydrogen sulfide and triisobutene are fully mixed and reacted with Amberlyst35 resin, the reaction pressure is 1.0Mpa, and the reaction temperature is 5°C; After 4 hours, the sampling analysis showed that the conversion rate of triisobutene was 71.01%. The selectivity of tert-butyl mercaptan in the product was 12.8%, the selectivity of tert-octyl mercaptan was 21.6%, and the selectivity of tert-dodecyl mercaptan was 65.6%. Other thiols are generated; the reaction product chromatographic analysis spectrum is shown in figure 1 .
Embodiment 2
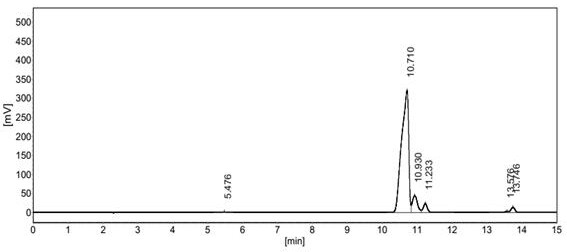
[0031] The reactor parameters were the same as those in Example 1, and the catalyst was also 30 mL of dry Amberlyst35 resin. The catalyst was treated in situ under nitrogen, room pressure, and 110° C. overnight, and hydrogen sulfide was controlled to flow into the reactor at 60 mL / min through a mass flow meter. Isobutene was fed into the reactor through a high-efficiency liquid phase pump at 12mL / h. After the hydrogen sulfide and triisobutene were fully mixed, they were contacted with Amberlyst35 resin for reaction. The selectivity of tert-butyl mercaptan in the product is 9.01%, the selectivity of tert-octyl mercaptan is 14.78%, the selectivity of tert-dodecyl mercaptan is 76.21%, no other mercaptans are generated, and the online reaction is 200h The catalyst showed no obvious signs of deactivation.
Embodiment 3
[0033] The reactor parameters are the same as those in Example 1. The catalyst is also 30 mL of dry Amberlyst35 resin. The catalyst is treated in situ under nitrogen, room pressure, and 110° C. overnight, and hydrogen sulfide is passed into the reactor at a rate of 65 mL / min through a mass flow meter. Diisobutene is fed into the reactor through a high-efficiency liquid phase pump at 15mL / h. After the hydrogen sulfide and diisobutene are fully mixed, they are contacted with Amberlyst35 resin for reaction. The rate is 93.6%, the selectivity of triisobutene in the product is 8.8%, the selectivity of tert-octyl mercaptan is 88.4%, the selectivity of tert-dodecyl mercaptan is 2.8%, and no other by-products are generated.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com