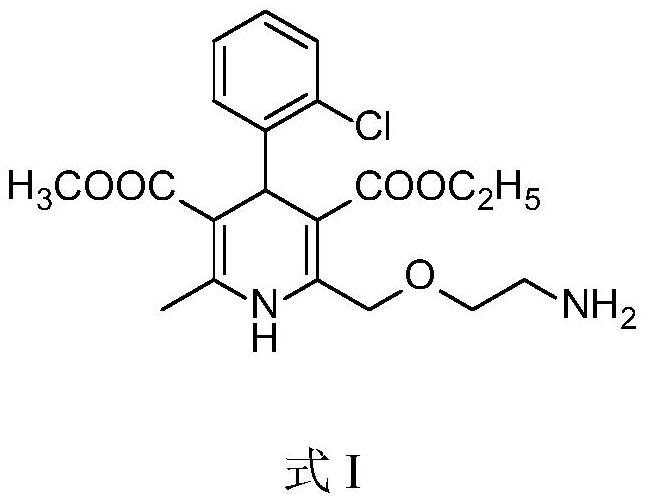
Method for preparing amlodipine besylate intermediate by using micro-reaction device
A technology of amlodipine besylate and micro-reaction device, which is applied in the direction of organic chemistry, can solve the problems of poor appearance, low conversion rate, and many side reactions of ring-closing products, and achieve good product quality, small reaction volume, The effect of short reaction times
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
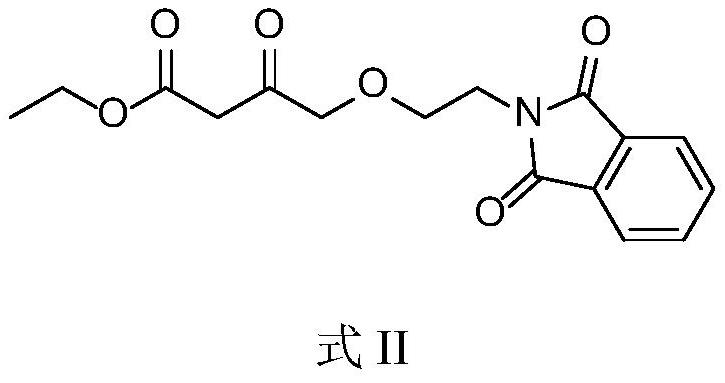
[0052] Preparation of β-dicarbonyl compounds: Dissolve 2-chloroethanol (1.61g, 20mmol) and potassium phthalimide (4.06g, 22.0mmol) in N,N-dimethylformamide (20ml) , stirred at 50-60°C, after TLC detected that the reaction was complete, added water (40ml) and ethyl acetate (40ml) to the reaction solution, stirred, separated the liquid, discarded the water phase, and used saturated brine (30ml x2 ) was washed twice, dried over anhydrous sodium sulfate, and concentrated to dryness under reduced pressure to obtain the crude product of the β-dicarbonyl compound intermediate, which was directly used in the next step. Dissolve the above crude product in N,N-dimethylformamide (20ml), add potassium tert-butoxide (2.46g, 22.0mmol) and stir at 20-25°C for about 15min, add ethyl 4-bromoacetoacetate (4.18 g, 20.0mmol), stirred at 20-25°C, after the reaction was detected by TLC, added water (40ml) and ethyl acetate (40ml) to the reaction solution, stirred, separated, discarded the aqueous p...
Embodiment 2
[0056] Refer to Example 1 for the preparation process of β-dicarbonyl compound.
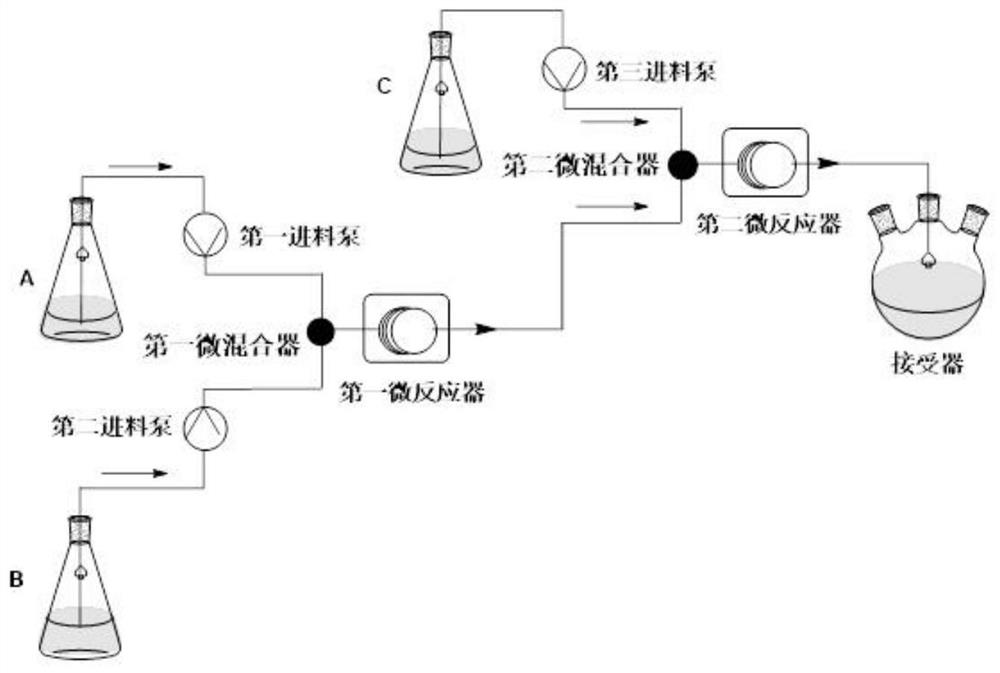
[0057] The above β-dicarbonyl compound (3.19g, 10.0mmol), piperidine (43mg, 0.5mmol) and acetic acid (30mg, 0.5mmol) were mixed, dissolved in ethanol (6.6ml), and stirred evenly to obtain a homogeneous solution a. Dissolve o-chlorobenzaldehyde (1.40g, 10.0mmol) in ethanol (6.6ml) and stir evenly to obtain a homogeneous solution C. The homogeneous solution A and the homogeneous solution C are pumped into the Y-shaped mixer at a flow rate of 0.250mL / min respectively, and after mixing, they are passed into the first microreactor with a coil inner diameter of 1mm. The volume of the reactor is 15mL. The temperature was controlled at 90° C., and the reaction residence time was 20 minutes.
[0058] At the same time, methyl 3-aminocrotonate (1.15 g, 10.0 mmol) was dissolved in ethanol (6.6 ml), and stirred evenly to obtain a homogeneous solution B. The homogeneous solution B is pumped into the T-type ...
Embodiment 3
[0060] Refer to Example 1 for the preparation process of β-dicarbonyl compound.
[0061] The above-mentioned β-dicarbonyl compound (3.19g, 10.0mmol), piperidine (213mg, 2.5mmol) and acetic acid (150mg, 2.5mmol) were mixed, dissolved in ethanol (6.6ml), and stirred evenly to obtain a homogeneous solution a. Dissolve o-chlorobenzaldehyde (1.40g, 10.0mmol) in ethanol (6.6ml) and stir evenly to obtain a homogeneous solution C. The homogeneous solution A and the homogeneous solution C are pumped into the Y-shaped mixer at a flow rate of 0.250mL / min respectively, and after mixing, they are passed into the first microreactor with a coil inner diameter of 1mm. The volume of the reactor is 15mL. The temperature was controlled at 90° C., and the reaction residence time was 20 minutes.
[0062] At the same time, methyl 3-aminocrotonate (1.15 g, 10.0 mmol) was dissolved in ethanol (6.6 ml), and stirred evenly to obtain a homogeneous solution B. The homogeneous solution B is pumped into...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com