Preparation method of imidapril hydrochloride
A technology of imidapril hydrochloride and alanine is applied in the field of preparation of imidapril hydrochloride, which can solve the problems of a large number of impurities and low purity, and achieve the effects of small impurities, control of product purity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] A preparation method for imidapril hydrochloride, comprising the following steps:
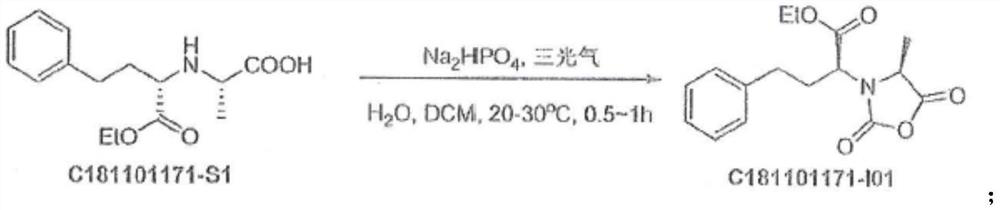
[0107] (1) Synthesis of N-[1-(S)-ethoxycarbonyl-3-phenylpropyl]-L-alanine-N-carboxy anhydride:
[0108] Mix and dissolve 3.5kg water and 1.02kg disodium hydrogen phosphate, mix with 2.62kg dichloromethane, 1kg N-[(S)-(+)-1-(ethoxycarbonyl)-3-phenylpropyl]-L propane Amino acid (C181101171-S1), 2kg of dichloromethane, triphosgene / dichloromethane: 0.37kg: 2.64kg of mixed liquid 1 was added to a 200L reaction kettle with stirring in turn, the temperature was controlled at 20°C and stirred for 0.5h, and samples were taken in HPLC Control, stop the reaction when N-[(S)-(+)-1-(ethoxycarbonyl)-3-phenylpropyl]-L-alanine≤5.0%, let it stand for liquid separation for 30 minutes, add 2.06 to the organic phase kg of 2mol / L hydrochloric acid solution and 2.24kg of 23.5% sodium chloride were washed separately, and left to separate for 30 minutes, then added 0.5kg of anhydrous sodium sulfate and stirred...
Embodiment 2
[0115] A preparation method for imidapril hydrochloride, comprising the following steps:
[0116](1) Synthesis of N-[1-(S)-ethoxycarbonyl-3-phenylpropyl]-L-alanine-N-carboxy anhydride:
[0117] Mix and dissolve 35kg of water and 10.2kg of disodium hydrogen phosphate, and 26.2kg of dichloromethane, 10kg of N-[(S)-(+)-1-(ethoxycarbonyl)-3-phenylpropyl]-L alanine Acid (C181101171-S1), 20kg of dichloromethane, triphosgene / dichloromethane: 3.7kg: 26.4kg of mixed solution 1 was added to a 200L reaction kettle with stirring in turn, temperature controlled and stirred at 25°C for 0.8h, sampling was performed in the HPLC control , N-[(S)-(+)-1-(ethoxycarbonyl)-3-phenylpropyl]-L-alanine ≤5.0% stop the reaction, let stand for liquid separation for 60 minutes, add 20.6kg organic phase 2mol / L hydrochloric acid solution and 22.4kg of 23.5% sodium chloride were washed separately and left to separate liquids for 60 minutes, added 5kg of anhydrous sodium sulfate and stirred for dehydration fo...
Embodiment 3
[0125] A preparation method for imidapril hydrochloride, comprising the following steps:
[0126] (1) Synthesis of N-[1-(S)-ethoxycarbonyl-3-phenylpropyl]-L-alanine-N-carboxy anhydride:
[0127] Mix and dissolve 70kg of water and 20.4kg of disodium hydrogen phosphate, and 52.4kg of dichloromethane, 20kg of N-[(S)-(+)-1-(ethoxycarbonyl)-3-phenylpropyl]-L alanine Acid (C181101171-S1), 40kg of dichloromethane, triphosgene / dichloromethane: 7.4kg: 52.8kg of mixed liquid 1 was added to a 200L reaction kettle with stirring in turn, the temperature was controlled at 30°C and stirred for 1.0h, and the sample was sampled by HPLC. , N-[(S)-(+)-1-(ethoxycarbonyl)-3-phenylpropyl]-L-alanine ≤5.0% stop the reaction, let stand for liquid separation for 90 minutes, add 41.2kg organic phase 2mol / L hydrochloric acid solution and 44.8kg of 23.5% sodium chloride were washed separately and left to separate liquids for 90 minutes, adding 10kg of anhydrous sodium sulfate and stirring for dehydration...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com